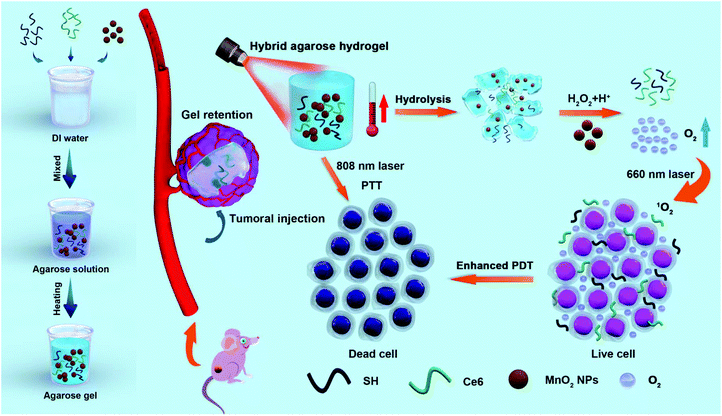
Responsive agarose hydrogel incorporated with natural humic acid and MnO2 nanoparticles for effective relief of tumor hypoxia and enhanced photo-induced tumor therapy†
Mengmeng
Hou‡
ab,
Weiwei
Liu‡
c,
Lei
Zhang
d,
Leiyang
Zhang
a,
Zhigang
Xu
 ab,
Yang
Cao
*c,
Yuejun
Kang
ab,
Yang
Cao
*c,
Yuejun
Kang
 ab and
Peng
Xue
ab and
Peng
Xue
 *ab
*ab
aKey Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, School of Materials and Energy, Southwest University, Chongqing 400715, China. E-mail: xuepeng@swu.edu.cn
bChongqing Engineering Research Center for Micro-Nano Biomedical Materials and Devices, Chongqing 400715, China
cChongqing Key Laboratory of Ultrasound Molecular Imaging, Institute of Ultrasound Imaging, Second Affiliated Hospital, Chongqing Medical University, Chongqing, 400010, China. E-mail: yangcao@cqmu.edu.cn
dInstitute of Sericulture and System Biology, Southwest University, Chongqing 400716, China
First published on 4th November 2019
Abstract
In spite of widespread applications of nano-photosensitizers, poor tumor penetration and severe hypoxia in the tumor microenvironment (TME) always result in an undesirable therapeutic outcome of photodynamic therapy (PDT). Herein, a biocompatible agarose-based hydrogel incorporated with sodium humate (SH), manganese oxide (MnO2) and chlorin e6 (Ce6) was synthesized as agarose@SH/MnO2/Ce6 through a “co-trapped” strategy during a sol–gel process and employed for combined photothermal therapy (PTT) and enhanced PDT. NIR-induced local hyperthermia is responsible for not only activating Ce6 release, but also triggering the catalytic decomposition of H2O2 mediated by MnO2 to relieve hypoxia. Such a hybrid hydrogel can realize deep tissue penetration through intratumoral injection, and exhibit remarkable tumor-site retention. Moreover, programmed laser irradiation led to an extremely high tumor growth inhibition rate of 93.8% in virtue of enhanced PTT/PDT. In addition, ultralow systemic toxicity caused by the hybrid hydrogel was further demonstrated in vivo. This reliable and eco-friendly hydrogel paves the way for the development of smart gel-based biomaterials, which respond to both exogenous and endogenous stimuli, towards the management of cancer and other major diseases.
Introduction
Cancer, as one of the most complicated and intractable diseases, has been seriously threatening human health and causing a huge reduction of life expectancy, attributed to the high morbidity and mortality rate in the past few decades.1–3 Surgery is the most commonly used approach to treat cancer by the manual removal of tumorous tissue.4 However, this therapeutic method is only valid for eliminating well-defined and primary tumors on non-vital organs or tissues. Moreover, potential bleeding and infections may impair patient compliance during surgery, and the rate of treatment failure is relatively high owing to the incidence of relapse.5,6 To reduce the high recurrence rate and incomplete eradication of solid tumors via surgery, chemotherapy and radiotherapy have been implemented as adjuvant treatment modalities.7,8 However, severe complications and adverse side effects always emerge in the process of these therapies owing to the limited selectivity and specificity of chemotherapeutic drugs and radioactive exposure. In this respect, there is an urgent need for developing promising therapeutic modalities with decent tumor specificity, high treatment efficacy and minimal adverse effects for clinical applications.Currently, tumor-targeted drug delivery techniques allow a desirable kinetics of drug release specifically to the tumor region with extended periods of time, which can effectively overcome the above-mentioned shortcomings of traditional methods.9,10 To date, many drug delivery systems have been successfully developed for the purpose of localized drug delivery, such as dendrimers,11 micelles,12 liposomes,13 nano/microparticles,14 hydrogel,15etc. Among them, injectable hydrogel is highly promising for intratumoral administration of both hydrophilic and hydrophobic autitumor drugs, which not only realizes sustained and controllable drug release but also minimizes side effects caused by systemic exposure.15–20 Polymeric hydrogel can be flexibly designed with high biocompatibility and degradability, benefiting the activity maintenance of the encapsulated drug. The release of the pharmaceutical ingredient from hydrogel can be activated by diffusion, swelling or environmental stimuli.21,22 Having been approved as a biocompatible polysaccharide by the US Food and Drug Administration (FDA), agarose is a purified linear and neutral galactan hydrocolloid extracted from agar or agar-bearing marine algae.23 Low melting point (LMP) agarose melts at 65.5 °C and sol-to-gel transition is initiated during the cooling process at a temperature below 25 °C. LMP agarose hydrogel has displayed enormous potential for on-demand localized drug administration, which can be precisely regulated by varying the ambient temperature.24–26 Therefore, functionalized LMP agarose hydrogel is anticipated to be rationally designed and developed for multimodal tumor therapy through one single injection, thereby avoiding the insufficient bioavailability of drugs and improving therapeutic outcome with high patient compliance.
Photothermal therapy (PTT) has been extensively studied as a minimally invasive tumor ablation strategy, in which the destruction of cancerous tissue is achieved based on local hyperthermia mediated by photothermal agents (PTAs) under near-infrared (NIR) light irradiation.27–30 Taking advantage of the simplicity, minimal invasiveness and high spatiotemporal precision, PTT can effectively improve the therapeutic outcome compared to traditional treatment approaches.29 Apart from eradicating tumor through thermal shock, light-responsive PTAs also serve as potential candidates for triggering on-demand drug release by simply modulating the stimuli.31,32 In particular, light-induced phase transition of drug-loaded hydrogel has received increasing attention for controlled drug delivery, which can be accurately adjusted by varying the operating parameters of the light source, such as laser wavelength, output power and irradiation time.33–35 Thus far, a good variety of PTAs have been developed to attain satisfactory PTT performance.36 However, the majority of them still possess limitations for clinical usage, such as complex synthesis, low biosafety and non-degradability. Humic acid (HA), as an organic substance extracted from biochemical humification of plant and animal matter, has recently attracted increasing interest for both PTT and photoacoustic (PA) imaging, attributed to its outstanding photothermal conversion capacity.37,38 Potential biohazard caused by HA is minimal towards a good variety of living organisms.39 Unlike nanoagents liable to aggregate in the non-aqueous phase, small molecules of HA are extremely prone to be uniformly dispersed when being encapsulated into hydrogel, which facilitates the maintenance of their photothermal stability and the homogeneous heat generation triggered by laser irradiation. Sodium humate (SH), as a sodium salt derivative, displays an improved solubility compared to HA while retaining the equivalent photothermal property of HA.
Meanwhile, photodynamic therapy (PDT) has been identified as an effective approach for cancer treatment both in preclinical trials and clinical applications, by utilizing the oxygen reactive species (ROS) produced from oxygen molecules in the presence of photosensitizers (PS) under light excitation.40,41 Chlorin e6 (Ce6), as a second generation of PS, has been commonly used for PDT owing to its high ROS quantum yield and low dark toxicity.42 In another aspect, intrinsic hypoxia inside the solid tumor has been regarded as one of the undesirable characteristics that promote tumor progression and metastasis, which originates from abnormal cell proliferation, aberrant vasculature, and dysfunctional lymphatic system.43,44 Thereby, hypoxia-associated resistance often occurs as a result of insufficient oxygen supply during the process of PDT, which severely compromises therapeutic efficacy during the treatment of large solid tumors.45–47 To modulate the hypoxic tumor microenvironment (TME), manganese dioxide (MnO2) incorporated nanostructures have exhibited high selectivity and specificity towards a sustained production of O2 from the decomposition of high-level endogenous H2O2 inside the tumor, which has been verified to be effective for the amelioration of hypoxia and favorable for enhanced PDT of the tumor.48–50
Herein, we innovatively proposed a “co-trapped” approach by simultaneously encapsulating SH, Ce6 and MnO2 nanoparticles (NPs) into LMP agarose, and the as-synthesized agarose@SH/MnO2/Ce6 hybrid hydrogel was successfully applied for enhanced PTT/PDT through an effective relief of tumor hypoxia (Fig. 1). The resulting hybrid hydrogel as an injectable material into the diseased lesion exhibits superiorities for tumor therapy: first, this hydrogel with acceptable biocompatibility and biodegradability can be introduced into the solid tumor, especially into the innermost region, through precise injection. Second, permeation of Ce6 and MnO2 NPs from the hydrogel matrix into the ambient environment was sustainable subject to the softening and hydrolysis of the agarose hydrogel, which can also be accelerated by the generation of local hyperthermia. Third, the hydrogel itself can be used for PTT, as SH acts as a light absorber that converts light into thermal energy under NIR light irradiation. Moreover, tumor hypoxia is intended to be effectively attenuated through oxygen generation from H2O2 decomposition catalyzed by MnO2. Thereafter, a tremendous enhancement of PDT is achieved upon the hydrogel being exposed to a 660 nm laser. The potential biohazard effects and clearance by the immune system are reduced to a minimal level, thanks to the remarkable retention of the hydrogel within the tumorous area but without entering the circulatory system, eventually achieving the objective of “one injection, multiple therapies”.
Experimental section
Materials
Humic acid sodium salt (sodium humate, SH), ultrapure LMP agarose, hydrogen peroxide (H2O2, 30%), dimethyl sulfoxide (DMSO) and 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 98%) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China). Potassium permanganate, 1,3-diphenyl-isobenzofuran (DPBF) and oleic acid (technical grade, 90%) were purchased from Sigma-Aldrich (MO, USA). Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1), fetal bovine serum (FBS), TrypLE™ Express Enzyme (1×), phosphate-buffered saline (PBS, 10×), 4′,6-diamidino-2-phenylindole (DAPI), calcein AM and propidium iodide (PI) were acquired from Thermo Fisher Scientific (MA, USA). 2,7-Dichlorofluorescein diacetate (DCFH-DA) was obtained from GEN-VIEW Scientific Inc. (CA, USA). Chlorin e6 (Ce6) was supplied by Frontier Scientific, Inc. (UT, USA). Hematoxylin and eosin (H&E) staining kit, one step TUNEL apoptosis assay kit, Ki67 cell proliferation kit, HIF-1α monoclonal antibody and Rat TNF-α ELISA Kit were purchased from Beyotime Biotechnology (Shanghai, China). All cell line types were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Female BALB/c mice and KM mice (6 weeks, ∼20 g) were obtained from Chongqing Teng Xin Bill Experimental Animal Sales Co., Ltd.
000 U mL−1), fetal bovine serum (FBS), TrypLE™ Express Enzyme (1×), phosphate-buffered saline (PBS, 10×), 4′,6-diamidino-2-phenylindole (DAPI), calcein AM and propidium iodide (PI) were acquired from Thermo Fisher Scientific (MA, USA). 2,7-Dichlorofluorescein diacetate (DCFH-DA) was obtained from GEN-VIEW Scientific Inc. (CA, USA). Chlorin e6 (Ce6) was supplied by Frontier Scientific, Inc. (UT, USA). Hematoxylin and eosin (H&E) staining kit, one step TUNEL apoptosis assay kit, Ki67 cell proliferation kit, HIF-1α monoclonal antibody and Rat TNF-α ELISA Kit were purchased from Beyotime Biotechnology (Shanghai, China). All cell line types were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Female BALB/c mice and KM mice (6 weeks, ∼20 g) were obtained from Chongqing Teng Xin Bill Experimental Animal Sales Co., Ltd.
Synthesis of MnO2 NPs
Honeycomb MnO2 NPs were synthesized through a typical reduction process.47,48 Briefly, KMnO4 (0.25 g) was dissolved in DI water (125 mL), and the solution was stirred at room temperature for 0.5 h. Then, oleic acid (2.8 mL) was introduced to form a steady emulsion, which was subsequently maintained at ambient temperature for 24 h until the formation of a dark brown product. The final product was harvested after rinsing with DI water and ethanol at least three times to completely remove the residual reactants. Finally, the as-synthesized MnO2 NPs were dried under vacuum at 60 °C overnight for further use.Preparation of the hybrid hydrogel
SH (500 mg) was dissolved in DI water (60 mL), and insoluble residues were eliminated through centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The collected supernatant was dialyzed for at least 3 days for purification. Then, the obtained solution containing SH was immediately frozen in liquid nitrogen and further dehydrated in a vacuum to harvest the black SH powder. To constitute the agarose@SH/MnO2/Ce6 hydrogel, LMP agarose (100 mg), MnO2 NPs (10 mg), purified SH powder (10 mg) and Ce6 (2 mg) were firstly dissolved in DI water (10 mL) to form a heterogeneous mixture. Then, a uniform dispersion was attained by melting the abovementioned mixture in a microwave oven. Finally, the agarose@SH/MnO2/Ce6 hydrogel was obtained subsequent to the previous dispersion being cooled down to room temperature. The composition of the as-synthesized agarose-based hybrid hydrogel remained constant for the follow-up studies if not otherwise stated.
000 rpm for 30 min. The collected supernatant was dialyzed for at least 3 days for purification. Then, the obtained solution containing SH was immediately frozen in liquid nitrogen and further dehydrated in a vacuum to harvest the black SH powder. To constitute the agarose@SH/MnO2/Ce6 hydrogel, LMP agarose (100 mg), MnO2 NPs (10 mg), purified SH powder (10 mg) and Ce6 (2 mg) were firstly dissolved in DI water (10 mL) to form a heterogeneous mixture. Then, a uniform dispersion was attained by melting the abovementioned mixture in a microwave oven. Finally, the agarose@SH/MnO2/Ce6 hydrogel was obtained subsequent to the previous dispersion being cooled down to room temperature. The composition of the as-synthesized agarose-based hybrid hydrogel remained constant for the follow-up studies if not otherwise stated.
Characterization of the hybrid hydrogel
A digital image of the agarose@SH/MnO2/Ce6 hydrogel was acquired by using a Nikon D810 digital camera. The element composition and morphology of MnO2 NPs were analyzed using JSM-7800F field emission scanning electron microscopy with energy dispersive X-ray spectroscopy (FESEM-EDX). X-ray diffraction (XRD) pattern of MnO2 NPs was recorded using an XRD-7000 X-ray diffractometer. The UV-vis-NIR absorption spectrum of MnO2 NPs was acquired using a UV-1800 UV-vis spectrophotometer. The dynamic shear rheological properties of the hybrid hydrogel were evaluated under shear conditions at a frequency of 1 Hz using a DHR-1 temperature-controlled rheometer. Briefly, a plate was lowered onto the sample with a nominal gap of ∼1 mm to the facing specimen holder. Temperature-sweep dynamic shear test was conducted in a temperature range from 30 to 60 °C with a ramp rate of 3 °C min−1.Photothermal property of the hybrid hydrogel
To investigate the photothermal conversion capacity of the agarose@SH/MnO2/Ce6 hydrogel, a quartz tube containing 1 mL hybrid hydrogel at various equivalent SH concentrations was irradiated using a NIR laser (808 nm, 1.5 W cm−2) for 10 min. The local temperature was dynamically monitored by using a digital thermometer, and real-time infrared thermal images were recorded using an infrared thermal imager (TiS55, Fluke, USA). Photothermal stability of the hybrid hydrogel was analyzed by periodic NIR irradiation for four cycles (laser on for 10 min per cycle), and the temperature variation was digitally measured using the same thermometer.NIR-triggered drug release in vitro
1 mL agarose@SH/MnO2/Ce6 hydrogel was firstly prepared in the bottom of a cuvette via a standard gelatinization process. Afterwards, 1 mL 1 × PBS was added into the same cuvette upon hydrogel formation. Then, the hybrid hydrogel was irradiated using a NIR laser (808 nm, 1.5 W cm−2) for a predesigned time period, and the released Ce6 in the supernatant was determined through fluorescence spectrophotometry. Specifically, the laser was switched on for 5 min and subsequently switched off for another 5 min during 60 min of treatment. Then, a liquid sample (200 μL) was obtained from the releasing system every 5 min, and the system was replenished with fresh medium at an equivalent volume. The amount of released Ce6 was quantified based on a standard curve of fluorescence intensity vs. standard Ce6 concentration (λex: 425 nm, λem: 660 nm) using a SPARK 10M microplate reader. In the meantime, the temperature change of the hybrid hydrogel was continuously monitored using an infrared thermal imager (TiS55, Fluke, USA). On the other hand, the release of MnO2 NPs from the hybrid hydrogel was measured by using inductively coupled plasma mass spectrometry (ICP-MS; XSeriesII, Thermo Scientific).Swelling and degradation behavior
To understand the swelling behavior, 1 mL of agarose@SH/MnO2/Ce6 hydrogel was immersed in 40 mL of 1 × PBS (pH = 7.4 or 6.5) at 37 °C or 60 °C. The initial weight of the hybrid hydrogel was measured as W0. At a predetermined time point, the hydrogel was taken out and weighed as W1 (hydrated weight). Then, the swelling ratio of the hydrogel was determined in accordance with formula (1).| Swelling ratio (%) = (W1 − W0)/W0 × 100% | (1) |
To characterize hydrogel degradation, the agarose@SH/MnO2/Ce6 hydrogel was collected from the medium at a predesigned time point and was immediately freeze-dried in liquid nitrogen, and its weight was subsequently measured as W2. The degradation rate is reflected by the weight loss percentage of the hydrogel after the drying process, which was calculated as formula (2).
| Degradation rate (%) = (W0 − W2)/W0 × 100% | (2) |
Oxygen generation efficiency in vitro
O2 generation capacity of the agarose@SH/MnO2/Ce6 hydrogel was determined in a closed chamber coupled with an oxygen probe from a JPB-607A portable digital LCD dissolved oxygen meter. Briefly, the hydrogel (1 mL) was immersed in deoxygenated 1 × PBS (10 mL) containing 100 mM H2O2, and NIR laser irradiation (808 nm, 1.5 W cm−2) was applied where applicable. The dissolved O2 level was monitored at 1 min intervals.Cellular uptake of Ce6 in vitro
Cellular internalization efficiency of Ce6 was studied through both laser scanning confocal microscopy (LSCM) and flow cytometry. Specifically, 4T1 cells (initial seeding density: 1 × 105 cells per well) were cultured in a 12-well plate at 37 °C overnight. After that, the cells were exposed to the agarose@SH/MnO2/Ce6 hydrogel (100 μL), which was immediately irradiated using a NIR laser (808 nm, 1.5 W cm−2) for 5 min. After another incubation for 0.5 or 2 h, the cells were fixed and stained with DAPI (1 μg mL−1) for 5 min, followed by examination under an LSM 800 confocal microscope. In another aspect, flow cytometry was conducted to quantitatively evaluate the cellular uptake of Ce6. After various treatments, the cells were resuspended in PBS and Ce6 fluorescence emission from individual cells was tracked using a NovoCyte flow cytometer. Data collected from the acquisition system were analyzed using the software FlowJo v10 (FlowJo, LLC, USA).Light-induced ROS generation in vitro
DPBF, as a fluorescent probe, was firstly used to evaluate the ROS generation efficiency of the agarose@SH/MnO2/Ce6 hydrogel during the photodynamic process. Briefly, 1 mL hybrid hydrogel was prepared in the bottom of a cuvette, followed by adding 1 mL PBS into the container. Thereafter, the hydrogel was exposed to a NIR laser (808 nm, 1.5 W cm−2) for 10 min. 1 mL of the supernatant was transferred into a new cuvette, and 5 μL of DPBF (1 mg mL−1, dissolved in DMSO) was added into the previous solution. Then, the mixture was irradiated using an optical laser (660 nm, 1 W cm−2) for 10 min, during which UV-vis absorption spectra were recorded at a time interval of 2 min. The decreasing rate of optical absorption intensity at 417 nm was calculated to reflect in situ ROS generation efficiency.DCFH-DA, as a cell-permeable fluorogenic probe, was utilized to measure intracellular ROS generation. Briefly, 4T1 cells were incubated in a 12-well plate (initial seeding density: 1 × 105 cells per well) overnight. Afterwards, the culture medium was displaced with 1 mL of the supernatant as previously mentioned in the DPBF assay. After incubation for 2 h, the cells were subjected to optical laser irradiation (660 nm, 1 W cm−2) for 10 min with or without H2O2 (100 μM). Subsequently, the cells were thoroughly rinsed with 1 × PBS, followed by staining with DCFH-DA (10 μM) for 1 h and DAPI (1 μg mL−1) for 5 min. In the end, fluorescence images of the stained cells were obtained using LSCM (LSM800, Zeiss, Germany).
Biocompatibility of the hybrid hydrogel in vitro
L929 fibroblasts and human umbilical vein endothelial cells (HUVECs) were utilized to assess the biocompatibility of the hybrid hydrogel. Specifically, a certain category of cells was cultured in a 96-well plate (initial seeding density: 1 × 104 cells per well) overnight. After discarding the previous medium, fresh culture medium (200 μL) containing the hybrid hydrogel at various concentrations was replenished into each well, followed by another incubation for 24 h. Next, the cells were rinsed with 1 × PBS, and were further treated with 200 μL MTT (0.5 mg mL−1). After 4 h of incubation, the supernatant was then discarded and DMSO (200 μL) was introduced into each well. Finally, cell viability was quantified based on the measurement of optical density (OD) (λ: 490 nm and 630 nm) using a SPARK 10 M microplate reader as indicated by formula (3). | (3) |
Cytotoxicity of the hybrid hydrogel in vitro
The murine mammary carcinoma cell line 4T1 and the human cervical carcinoma cell line HeLa were utilized for investigating the hybrid hydrogel-induced cytotoxicity in vitro. Specifically, tumor cells in a 12-well tissue culture plate (seeding density: 1 × 105 cells per well) were cultured at 37 °C overnight. Then, 200 μL of diversified hydrogel was introduced into each well, and the total volume of the medium was kept constant at 1 mL per well. Afterwards, the cells were subjected to the following treatments: DMEM (group 1), 808 nm + 660 nm laser (group 2), 100 μM H2O2 (group 3), agarose@SH + 808 nm laser (group 4), agarose@Ce6 + 660 nm laser (group 5), agarose@SH/Ce6 + 808 nm + 660 nm laser (group 6), agarose@SH/MnO2/Ce6 (group 7), agarose@SH/MnO2/Ce6 + 808 nm + 660 nm laser (group 8), and agarose@SH/MnO2/Ce6 + H2O2 + 808 nm + 660 nm laser (group 9). The H2O2 concentration was set at 100 μM for groups 3 and 9. For laser irradiation groups, 808 nm laser illumination (1.5 W cm−2) was performed for 10 min, followed by a further 2 h of incubation. Subsequently, 660 nm laser illumination (1.5 W cm−2) was switched on for 10 min, followed by another 2 h of incubation. Afterwards, the supernatant was discarded and the cells were gently rinsed with 1 × PBS at least three times. Then, cell staining was carried out by incubating the cells with a mixture of calcein AM (1 μg mL−1) and PI (1 μg mL−1) for 15 min, and the cells were then examined under a fluorescence microscope (IX73, Olympus, Japan). On the other hand, quantitative cell viability was measured using the standard MTT cell viability assay. Tumor cells in a 96-well tissue culture plate (seeding density of 1 × 104 cells per well) were cultured at 37 °C overnight. Then, 50 μL of diversified hydrogel was introduced into each well, and the total volume of the medium was kept constant at 200 μL per well. Thereafter, the cells received the same treatments as mentioned above with live/dead cell staining. Complying with a typical MTT protocol, cell viability in each well was calculated based on formula (3).Tumor model establishment in vivo
All animal studies complied with the guidelines and policies of the National Guide for Care and Use of Laboratory Animals (China), and were approved by the Institutional Animal Care and Use Committee (IACUC) of Southwest University. Xenografted tumors were built on female BALB/c mice (6 weeks, ∼20 g each) by subcutaneous inoculation with 4T1 cells (1 × 106 in 100 μL saline) onto their flank dorsal region upon anesthetization with isoflurane. Afterwards, all the mice were continuously bred for ∼10 days until the tumor size reached up to ∼150 mm3. The dimension of the tumors was manually measured using calipers, and the tumor volume was calculated according to the following formula (4).| Tumor volume = (longest diameter) × (shortest diameter)2 × 0.5. | (4) |
Photoacoustic/fluorescence (PA/FL) imaging in vivo
To demonstrate the PA imaging property, the agarose@SH/MnO2/Ce6 hydrogel was firstly prepared at various SH concentrations (31.25, 62.5, 125, 250, 500 μg mL−1). Then, PA images were acquired in vitro using a Vevo LAZR photoacoustic imaging system (λex = 725 nm, VisualSonics Inc., Canada) and quantitatively analyzed. In another aspect, tumor bearing BALB/c mice were treated with 50 μL of hybrid hydrogel through intratumoral injection, and PA imaging of the tumor region was performed at 1 h post-injection using the abovementioned PA imaging platform. For fluorescence imaging in vivo, tumor bearing BALB/c mice were intratumorally administered with the hybrid hydrogel or free Ce6 (equivalent Ce6 concentration: 0.5 mg kg−1). At 0, 2, 6, 24, 72 and 168 h post-injection, fluorescence images of the mice were recorded using a Fusion imaging system (λex = 630 nm, λem = 670 nm, Fusion FX7 Spectra, VILBER, France). In the meantime, the mice were euthanized to collect the tumors and major organs for ex vivo imaging via the same Fusion imaging system.Tumor inhibition in vivo
BALB/c mice bearing 4T1 tumors were randomly allocated into 10 groups (n = 4 for each group): (1) saline, (2) laser (808 nm + 660 nm); (3) agarose@Ce6; (4) agarose@Ce6 + 660 nm; (5) agarose@SH; (6) agarose@SH + 808 nm; (7) agarose@SH/Ce6; (8) agarose@SH/Ce6 + 808 nm + 660 nm; (9) agarose@SH/MnO2/Ce6; and (10) agarose@SH/MnO2/Ce6 + 808 nm + 660 nm. The mice in all groups were intratumorally injected with 50 μL hydrogel, followed by gelatinization for 1 h. Next, the tumor region was irradiated by NIR laser irradiation (808 nm, 1.5 W cm−2) for 10 min, and thermographic mapping of the mouse was concurrently performed using a thermal imaging camera (Ti55, Fluke, USA). One hour later, the tumor site was further exposed to an optical laser (660 nm, 1 W cm−2) for 10 min where applicable. Tumor volume and mouse body weight were continuously monitored for two weeks post-treatment. The tumor growth inhibition (TGI) rate was calculated on the basis of formula (5)| TGI = (1 − VT/Vc) × 100% | (5) |
On day 14, mice from all groups were sacrificed to harvest tumors. After being weighed, solid tumors were fixed with 10% formalin and paraffin-embedded, followed by slicing into thin sections (thickness: 5 μm) that were further stained with H&E, TUNEL, Ki67 and HIF-1α antibody according to the manufacturer's protocol. All histological sections were observed under an IX73 fluorescence microscope.
Biosafety evaluation
On day 14 post-treatment, mice from all groups were sacrificed to collect the vital organs. All these tissues were sliced into sections (thickness: 5 μm), which were further stained with H&E to evaluate the morphological features of necrosis. Moreover, routine blood tests were performed on KM mice subjected to an intratumoral injection of the hybrid hydrogel (50 μL) into the flank dorsal region, and whole blood (200 μL) was collected from the orbital venous plexus at day 0, 1, 7 and 14 post-treatment. The major index of blood was assessed using a hematology analyzer (BC-2600Vet, Mindray, China). Potential systemic inflammation after intratumoral injection of the hybrid hydrogel (50 μL) was explored by quantifying the TNF-α (an inflammatory marker) level in the peripheral blood. The supernatant containing blood serum was collected by centrifugation of whole blood (100 μL) at 3000 rpm for 10 min, after which the TNF-α level was determined using the Rat TNF-α ELISA Kit.Statistical analysis
All data were presented as mean ± SD using one-way analysis of variance (ANOVA) using OriginPro 8.5 software (OriginLab, MA, USA). A p-value less than 0.05 (*p < 0.05, n = 4) was considered to be statistically significant.Results and discussion
Characterization of MnO2 NPs
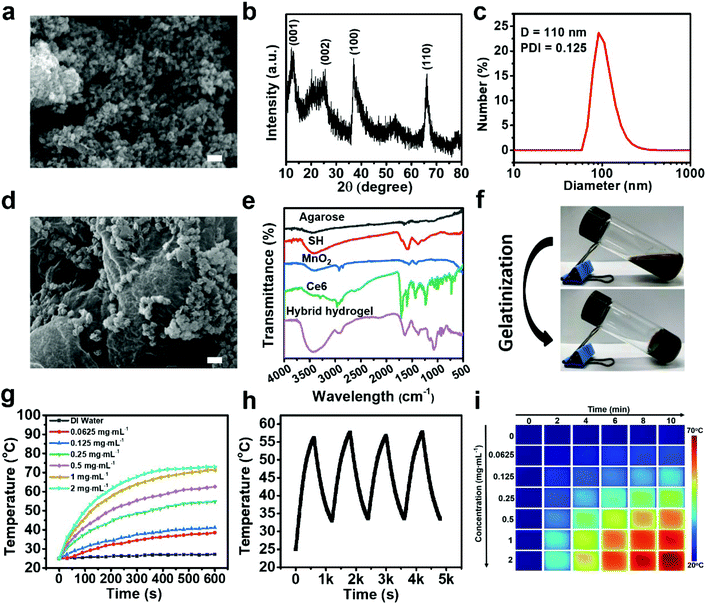
The synthesis of MnO2 NPs was performed via a simple soft chemistry through the reduction of KMnO4 at ambient temperature. The morphology and structure of MnO2 NPs were elucidated by SEM imaging (Fig. 2a). Clearly, MnO2 NPs exhibited a honeycomb structure with a diameter of ∼97 nm, which could be self-assembled from MnO2 nanoplatelets as revealed in the previous studies.51,52 To evaluate the elemental composition of MnO2 NPs, FESEM-EDX analysis was performed on a single particle. An intense signal corresponding to Mn and O was detected as evidenced by elemental mapping, suggesting their main chemical composition (Fig. S1†). Moreover, the crystallographic phase of MnO2 NPs was determined by XRD (Fig. 2b). Strong peaks were recorded at 2θ = 12.3, 24.3, 36.6, and 65.7°, which could be indexed to the (001), (002), (100) and (110) planes of birnessite-type MnO2, respectively.53 Additionally, the FT-IR spectrum of MnO2 NPs is illustrated in Fig. S2.† Characteristic peaks at 578 and 519 cm−1 are assigned to Mn–O stretching vibrations, and infrared bands at 3450 and 1640 cm−1 are ascribed to O–H vibrations. The bands at 1421 and 2935 cm−1 are indexed to the C–H bending or stretching vibrations of oleic acid.54 Furthermore, compared to the KMnO4 precursor, MnO2 NPs displayed a broad absorption band ranging from 250 to 500 nm, which corresponds well with the optical properties of these reported MnO2 nanomaterials (Fig. S3†).55 The hydrodynamic size of MnO2 NPs was measured as 110 nm with a polydispersity index (PDI) of 0.125, manifesting the formation of a uniform dispersion (Fig. 2c). In another aspect, decent aqueous dispersity, high performance of photothermal conversion and good biocompatibility of SH have been demonstrated in previous studies.26Characterization of the hybrid hydrogel
High homogeneity agarose@SH/MnO2/Ce6 hydrogel is significantly dependent on a thorough stirring of the corresponding liquid mixture at a temperature above the melting point of LMP agarose. SH, Ce6 and MnO2 NPs can be uniformly encapsulated into the cross-linked agarose matrix during the gelatinization process. The morphology of the as-developed hybrid hydrogel is revealed by the SEM image in Fig. 2d. Obviously, MnO2 NPs were effectively decorated onto the surface of the agarose matrix, and the loose structure of the hybrid hydrogel was incredibly favorable for liquid adsorption and follow-up dissolution. The FT-IR spectra of the hybrid hydrogel and its components are exhibited in Fig. 2e. Distinct peaks of 2890 and 1150 cm−1 from Ce6 denote the stretching vibration of the C–H and C–O–C groups, respectively.56 The FT-IR spectra of SH confirm the presence of numerous chemical groups, including hydroxyl, carboxyl and vinyl groups.26 As for agarose, a broad absorption band at 3400 cm−1 is associated with O–H stretching. Characteristic bands at 773, 894, and 932 cm−1 are ascribed to the 3,6-anhydro-β-galactose skeletal bending. The absorption peak at 1072 cm−1 is attributed to the deformation mode of C–O.57 The FT-IR spectrum of the hybrid hydrogel displayed all representative bands of agarose, Ce6, SH and MnO2 NPs, verifying a successful preparation of the final product. Moreover, the hybrid hydrogel exhibited a black color, which was in accordance with the precursor solution before gelatinization (Fig. 2f).Photothermal response of the hybrid hydrogel
At the beginning, we evaluated the photothermal property of purified SH upon NIR laser irradiation for 10 min (Fig. S4a†). As expected, the temperature increase of the SH solution was positively correlated to the agent concentration and laser irradiation time. In particular, the temperature of SH (1 mg mL−1) was increased from the initial 25 °C to 65.2 °C after 10 min of laser irradiation, suggesting a high performance of SH-mediated photothermal conversion. In parallel, the response of the hybrid hydrogel toward NIR laser illumination was similarly explored at various equivalent SH concentrations (Fig. 2g). Likewise, intense hyperthermia generation was effectively triggered by light irradiation, which is adequate for damaging tumor cells through the destruction of intracellular proteins and genetic materials. Interestingly, an even higher temperature increase was found in the hybrid hydrogel compared to free SH upon receiving laser irradiation for the same period, which could be interpreted as slower heat dissipation from the hydrogel attributed to attenuated liquid evaporation (Fig. S4b†). To investigate the photothermal stability of the hybrid hydrogel, periodic NIR laser irradiation was conducted for four cycles. There was no significant decrease of the peak temperature in each cycle, implying a satisfactory stability for light-induced PTT (Fig. 2h). Infrared thermography of the hybrid hydrogel was further performed to dynamically monitor the temperature during laser irradiation, and the data exhibited good agreement with the measurements using a digital thermometer (Fig. 2i).In vitro drug release
A facile route was proposed to investigate Ce6 release from the hybrid hydrogel, ingeniously avoiding the use of traditional dialysis bags. Briefly, the solidified hydrogel was formed in the bottom of a cuvette, and the drug content in the liquid supernatant was quantified after various treatments (Fig. 3a). We firstly evaluated the role that temperature played in regulating drug release. A more rapid Ce6 release was found at a higher temperature of 60 °C, in comparison with that at 37 °C under physiological conditions (Fig. 3b). Hyperthermia-responsive drug release can be ascribed to enhanced hydrogel hydrolysis and drug diffusion as indicated in the previous studies.24–26 Inspired by the positive correlation between temperature and drug dissolution, we further explored the effect of laser irradiation on Ce6 release. The fluorescence spectrum of the supernatant in each cuvette (λex = 405 nm) was measured after a specific period of NIR irradiation (808 nm, 1.5 W cm−2). A sustained Ce6 release was observed in the process of laser irradiation for 60 min, as evidenced by a time-dependent increase of fluorescence emission intensity at 662 nm, implying a good opportunity to use NIR light as an exogenous stimulus to regulate Ce6 diffusion (Fig. 3c, S5†). In addition, the release of MnO2 NPs can also be accelerated under NIR laser irradiation in comparison with the group without any treatment (Fig. S6a†).To further investigate the NIR-triggered unloading of the drug cargo, the hybrid hydrogel was periodically irradiated using a NIR laser in an “ON/OFF” fashion (5 min on and 5 min off per cycle). The temperature of the hybrid hydrogel rapidly rose to ∼50 °C after laser irradiation for 5 min, caused by the high performance of SH in photothermal conversion (Fig. 3d). Moreover, Ce6 release was considerably accelerated in the course of laser irradiation compared to that without any treatment (Fig. 3d, S6b†). Moreover, the profile of temperature variation and responsive Ce6 release was not remarkably changed during all five irradiation cycles, implying the potential for recycling in practical applications. To be more specific, the average Ce6 release rate during five consecutive ON/OFF laser irradiation cycles was calculated as shown in Fig. 3e. As anticipated, laser irradiation could effectively initiate a more rapid Ce6 release from the hybrid hydrogel. Notably, a relatively higher release rate in the first irradiation cycle could be ascribed to the dissolution of Ce6 on the surface or in the external layer of the hydrogel. By contrast, a moderate drug release was observed in the following irradiation cycles, owing to slower drug dissolution from the interior hydrogel. In another aspect, re-gelatinization of the hydrogel instantly occurred when switching off the laser, thereby restraining the encapsulated cargoes from releasing.
To elucidate in depth the underlying mechanism of Ce6 release, various hybrid hydrogels were fabricated by varying the concentrations of SH, DOX and LMP agarose. Obviously, cumulative Ce6 release was evidenced to have a positive correlation with the loading amount of SH and Ce6 in the hydrogel. A larger amount of encapsulated SH may render a stronger local hyperthermia during the same period, which resulted in faster drug release from the hydrogel (Fig. S7a and b†). Furthermore, the hybrid hydrogel prepared from a higher proportion of agarose content may lower the Ce6 release rate, which can be interpreted as the blockage of the Ce6 release pathway in a more densely crosslinked network (Fig. S7c†). As a result, follow-up hydrolysis and degradation could also be impeded when subjected to laser irradiation. Apart from the abovementioned factors related to the ingredient composition of the hybrid hydrogel, Ce6 release can also be accelerated by increasing the laser power density, implying that higher NIR laser power may facilitate a rapid disruption of the compact polymeric matrix and further trigger an accelerated dissolution of entrapped Ce6 (Fig. S7d†). To sum up, there exists appealing potential to customize the Ce6 release behavior from the hybrid hydrogel via the optimization of its chemical composition and the adjustment of the operating parameter of the NIR laser.
Rheological property and in vitro degradation
To investigate the thermoresponsive behavior of the hybrid hydrogel developed from 1% agarose, the rheological property was characterized through temperature sweeping analysis, where the change in storage modulus (G′) and loss modulus (G′′) was recorded over a temperature ranging from 30 °C to 60 °C (Fig. 3f). Both G′ and G′′ values decreased upon heating, suggesting a typical reduction of noncovalent crosslinking with increasing temperature. An intersection of G′ and G′′ curves was found at a temperature of ∼60 °C, indicating a gel–sol transition above the physiological temperature. The macromolecular chain disentanglement was dynamically slow as G′ was always higher than G′′ before phase transformation. We assume that the agarose concentration may play an important role in determining its thermoresponsive behavior, and thus temperature swelling curves of the hybrid hydrogel with 0.5% and 2% agarose were determined for comparison (Fig. S8†). G′′ was considerably larger than G′ for the gel prepared from 0.5% agarose, explicitly implying a liquid form of the mixture. Conversely, G′′ was an order of magnitude lower than G′ for 2% agarose hydrogel, manifesting its existence in solid state. Hence, the concentration of agarose for hybrid hydrogel synthesis was optimized as 1% for the following studies both in vitro and in vivo. The degradability of the hydrogel was assessed by calculating the swelling ratio and weight loss of the hybrid hydrogel in 1 × PBS. An elevated swelling ratio of the hydrogel was found at a relatively high incubation temperature (60 °C), further leading to an accelerated hydrolysis of the hydrogel as indicated by a more rapid weight loss under the same conditions (Fig. S9†). Thermal-induced acceleration of degradation can be ascribed to the rapid hydrolysis of long polysaccharide chains and subsequent slow-paced hydrolysis of the fragmented chains into soluble oligosaccharides.58 In another aspect, we found that the pH value of the incubating medium exhibited an insignificant impact on the swelling ratio and weight loss of the hybrid hydrogel.Cellular uptake of the released Ce6 in vitro
Effective uptake and intracellular internalization of Ce6 released from the hybrid hydrogel are highly desirable for enhanced PDT because the as-generated ROS directly induces damage to the mitochondrial respiratory chain. In this study, 4T1 cells were exploited to study the cellular uptake of Ce6 by tracking its green fluorescence using LSCM and flow cytometry (Fig. 4). Obviously, a typical time-dependent cellular uptake of Ce6 was evidenced by a higher fluorescence intensity when the incubation time was increased from 0.5 to 2 h. Notably, NIR laser irradiation further increased the intracellular localization of Ce6, in sharp contrast to the control group in the absence of irradiation (Fig. 4a). Light-enhanced cellular uptake of Ce6 can be interpreted as a higher level of available Ce6 in the culture medium, attributed to an accelerated drug release from the hybrid hydrogel under local hyperthermia as previously verified. Besides, mild local hyperthermia may also facilitate the cellular uptake of small drug molecules by an increase in the fluidity of the cell membrane as demonstrated in the previous studies.59,60 Quantitative results obtained from flow cytometry similarly indicated a NIR-enhanced cellular uptake of Ce6, and the uptake efficiency notably reached up to 72.88% in the group of “2 h plus laser” (Fig. 4b–d).H2O2 decomposition and enhanced ROS generation in vitro
MnO2 has been recognized as a prestigious inorganic catalyst that can activate the decomposition of H2O2 into O2, which is known to relieve hypoxia and improve the outcome of PDT. In this respect, the O2 generation performance was evaluated by measuring the level of dissolved O2 after incubating the hybrid hydrogel in H2O2 solution (Fig. 5a). We found that the MnO2-incorporated hydrogel effectively initiated a rapid O2 production in the first 15 min of incubation compared to other control groups. Subsequent O2 increase was not remarkable, which could be explained as the saturation of dissolved oxygen. Furthermore, a more rapid O2 generation was observed in the group of hybrid hydrogel plus NIR laser irradiation. We deduce that the laser-activated acceleration of gel hydrolysis may introduce more H2O2 into the polymeric matrix, which simultaneously reacted with the incorporated MnO2 NPs to lead to O2 generation.Oxygen in the triplet state can be converted into singlet oxygen (1O2), a category of cytotoxic ROS to destroy tumor cells, during the light-induced photodynamic process. The 1O2 producing ability mediated by the hybrid hydrogel was firstly evaluated using a DPBF probe, which is a non-selective probe for the detection and quantitative determination of ROS. As shown in Fig. 5b, H2O2 alone can render a certain degree of DPBF absorption decrease at 417 nm. In sharp contrast, a more remarkable absorbance decrease was observed in both hydrogel groups plus NIR laser irradiation, verifying that effective NIR-triggered release of the Ce6 photosensitizer is a prerequisite for efficient follow-up ROS generation. Notably, the highest amount of ROS was achieved in the hybrid hydrogel with MnO2 NP incorporation, on account of the amelioration of the dissolved O2 level through catalytic decomposition of H2O2. Alternatively, the intracellular 1O2 yield mediated by the hydrogel was analyzed using a DCFH-DA probe, which can be oxidized by ROS to generate a highly fluorescent substance DCF (Fig. 5c). Compared to the groups in the absence of NIR irradiation, there was a vivid green fluorescence signal representing ROS upon the hydrogel receiving NIR irradiation, suggesting that hyperthermia-induced hydrogel degradation is essential for Ce6 release to trigger PDT. Likewise, the strongest ROS generation was distinctly found in the group of hybrid hydrogel plus NIR laser, which agreed well with the previous findings of the 1O2 yield in the solution system. To quantitatively study the DCF fluorescence inside the cells, flow cytometry was performed and the results were in accordance with fluorescence microscopy (Fig. S10†).
In vitro cytotoxicity of the hybrid hydrogel
Satisfactory biocompatibility is critical for the successful application of biomaterials. SH alone would cause negligible cell death, which has been explicated in our previous study.26 Thereby, the cytotoxicity of MnO2 NPs and the hybrid hydrogel was evaluated on normal somatic cells, including L929 fibroblasts and HUVECs, via the MTT cell viability assay. After incubation with MnO2 NPs and the hybrid hydrogel for 24 h, the percentage of live cells was both above 90% even at a concentration as high as 2 mg mL−1, manifesting a good biocompatibility for clinical use (Fig. 6b and c). We thereby measured the cytotoxicity of the hybrid hydrogel with 4T1 murine breast cancer cells. The standard live and dead cell fluorescence staining indicated that hybrid hydrogel plus 808 and 660 nm laser (groups 8 and 9) induced the most outstanding tumor cell destruction, as illustrated by the strong red fluorescence signal in the PI channel (Fig. 6a). It is worth noting that a more severe cell ablation was observed in the presence of H2O2, which can be ascribed to enhanced ROS production at a higher level of oxygen. Quantitative cytotoxicity induced by the hybrid hydrogel was further measured by the MTT assay (Fig. 6d). The data present a similar trend with the corresponding observations in previous fluorescence microscopy. In sharp contrast to the control groups, more than 85% of the tumor cells were eradicated in group 9, implying a remarkable tumor ablation effect through enhanced PTT/PDT in vitro.In vivo retention of the hybrid hydrogel
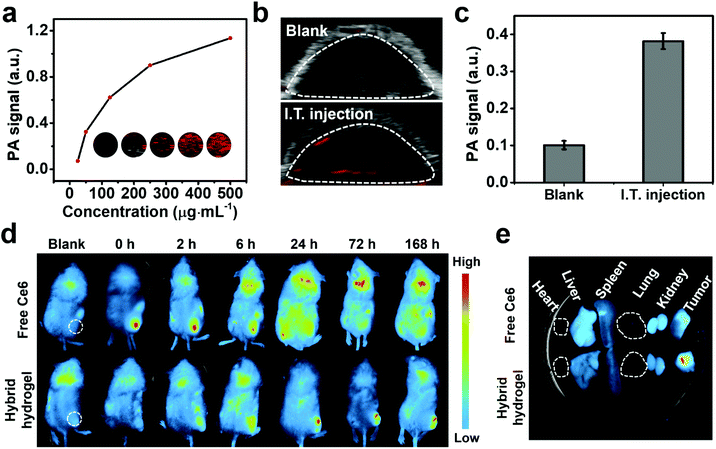
In vivo retention of the hybrid hydrogel was firstly evaluated through PA imaging. A previous study has proved that dynamic contrast-enhanced PA imaging can be realized on stimuli-responsive photothermal hydrogel.61 Therefore, we assume that PA imaging with high resolution and deep tissue penetration could be achieved by the as-synthesized hybrid hydrogel featured with high photothermal conversion performance. To evaluate the PA properties of the hybrid hydrogel, the amplitudes of the PA signal at a hydrogel concentration ranging from 25 to 500 μg mL−1 were measured in vitro (Fig. 7a). An intense PA signal was produced under laser excitation, and the signal intensity (average value within the pseudocolor region) strictly followed a concentration-dependent pattern. We thereby explored hydrogel retention after intratumoral injection by collecting the PA signal in the tumor area. After 1 h post-injection, a detectable PA signal was locally recorded, which was in striking contrast to the blank control group (Fig. 7b and c). These results clearly indicated that the hybrid hydrogel can be successfully administered with good retention through intratumoral injection, and may also benefit high performance and long-term PA monitoring in vivo.In addition, biodistribution of Ce6 after intratumoral injection of the hybrid hydrogel was characterized by tracking Ce6 fluorescence using an animal imaging system, and free Ce6-injected mice served as the control group (Fig. 7d and e). Free Ce6 exhibited instant tumoral accumulation and rapidly diffused throughout the mouse body within 6 h owing to blood circulation. Moreover, a rapid decay of Ce6 fluorescence within the tumor region was initiated at 2 h post-injection, suggesting a poor retention at the injection site. By contrast, the fluorescence signal of Ce6 was gradually intensified in the tumorous tissue within 24 h after hydrogel administration, which can be attributed to its long retention in the tumor area and a sustained cargo release from the hydrogel (Fig. S11†).
In vivo antitumor effect
The antitumor effect of the hybrid hydrogel was evaluated on BALB/c mice bearing 4T1 tumors subjected to diverse treatments. After intratumoral injection of various therapeutic agents and allowing for 1 h of gelatinization, the NIR-induced photothermal effect on the mouse body was dynamically monitored by thermographic imaging (Fig. 8a and b). A dramatic temperature increase was observed in all hydrogel groups after 5 min of NIR irradiation, indicating that the incorporation of SH can efficiently facilitate the conversion of light energy into local hyperthermia. Moreover, the final temperature exceeded 50 °C, which is higher than the threshold of 48 °C for efficacious PTT.62 Then, the mice were randomly assigned into ten groups. The tumor volume in each group was dynamically measured using a caliper for 14 days after various treatments (Fig. 7c). Compared to the saline group, single-modal treatment of PDT (group 4) or PTT (group 6) led to a moderate tumor inhibition effect after 14 days. By contrast, predominant tumor inhibition with a TGI of 65.8% was observed in the mice administered with agarose@SH/Ce6 plus laser irradiation (group 8), owing to a strong combined PTT/PDT effect. Furthermore, the most effective tumor eradication with a TGI as high as 93.8% was achieved upon laser irradiation mediated by the agarose@SH/MnO2/Ce6 hydrogel (group 10). We speculate that the component of MnO2 was responsible for the amelioration of hypoxia in the tumor microenvironment, which further resulted in improved PDT in vivo. Such tumor variation was also verified by the smallest anatomical size (Fig. 7d) and average weight of the dissected tumors among all groups at day 14 post-treatment (Fig. 7e). In addition, the weight loss of mice was not distinct regardless of any treatment, suggesting minimal adverse effects caused by these drug formulations (Fig. 7f).Histological analysis and biosafety evaluation
Tumor tissue destruction was evaluated by immunostaining of the excised tumor sections using anti-HIF-α, H&E, Ki67 and fluorometric TUNEL assays (Fig. 9). Hypoxia-inducible factor-1α (HIF-α) immunofluorescence staining was firstly used to evaluate the oxygen level inside the tumoral tissues. In contrast to other treatment groups, Nile red fluorescence was not observed from the tumor slices in group 10 (hybrid hydrogel + dual-laser), indicating an effective local hypoxia amelioration thanks to MnO2-mediated catalytic conversion of endogenous H2O2 into O2 (Fig. 9a). Such oxygenation in the tumor microenvironment is particularly favorable for attenuation of hypoxia-associated resistance during PDT. Moreover, typical pyknosis, karyorrhexis and karyolysis were further observed in the H&E stained slices from group 10, whereas intact membrane and nucleus were almost retained in the tumor cells from other control groups (Fig. 9b). Likewise, tumor cells expressing a high level of Ki67 were dramatically decreased in group 10, manifesting a significant weakening of cell proliferation (Fig. 9c). In the meantime, TUNEL-positive tumor cells considerably increased in the same group, implying a potent tumor killing effect by inducing cell apoptosis and necrosis. These histopathological findings verified a strong therapeutic outcome against 4T1 solid tumors through photo-activated therapy mediated by the hybrid hydrogel. The potential systemic toxicity of the hybrid hydrogel was assessed through blood routine test and histopathological analysis. The complete blood counts and other key hematology parameters of mice were not dramatically changed in two weeks after administration (Fig. S12†). Moreover, there were no apparent histological abnormality and lesion at the cellular or tissue level, as revealed by H&E stained slices of the major organs at day 14 post-treatment, which implied minimal systemic toxicity or adverse effect for in vivo applications (Fig. S13†). To further assess the immunogenicity of the hybrid hydrogel, the level of TNF-α in the blood serum of mice was determined by ELISA (Fig. S14†). The data fluctuation was insignificant during 14 days after intratumoral injection, indicating a weak immunogenicity induced by the hybrid hydrogel.Genetic alterations of tumor cells always lead to hyperplasia, uncontrolled proliferation, resistance to apoptosis and anaerobic glycolysis, which further produce a unique TME of hypoxia, oxidative stress and acidosis. In particular, aberrant vascularization and a poor blood supply generally result in permanent or transient hypoxia in the extracellular matrix of the tumor tissue.63 Unfortunately, hypoxia would cause an undesirable resistance to medical therapies, such as radiotherapy, chemotherapy and PDT. Perfluorocarbon-based oxygen shuttles have been extensively used to transport oxygen into the tumor. However, this strategy suffers from limited oxygen delivery efficiency, particularly toward the deep tumor region that is far away from the intratumoral vessels.64 Alternatively, MnO2 nanostructures can react with the high level of intrinsic H2O2 to sustainably produce O2, in order to enhance the efficacy of PDT, and the catalysis includes the following reactions:
| 2MnO2 + H2O2 = 2MnOOH + O2 | (6) |
| 2MnOOH + 4H+ + H2O2 = 2Mn2+ + 4H2O + O2 | (7) |
| 2MnOOH + 2H+ = MnO2 + Mn2+ + 2H2O. | (8) |
In this study, we have synthesized a category of honeycomb MnO2 NPs, which were used to relieve local hypoxia. This synthesis process is rapid through a series of mild reactions. Moreover, MnO2 NPs exhibited a high aqueous dispersity, which guarantees their uniform distribution within the hydrogel. After being encapsulated into the hydrogel, it is not essential to release MnO2 NPs from the gel matrix and perform their catalytic functions. In fact, interstitial fluid containing a high level of H2O2 can rapidly infuse into the hydrogel to react with MnO2 NPs during the hydrolysis process. Subsequently, the generated O2 can be released from the hydrogel and further diffuse into the tumor cells to increase the intracellular O2 level. On the other hand, we also demonstrated the release of Ce6 from the hydrogel with or without NIR laser activation. In the meantime, a highly efficient cellular uptake of Ce6 and the resultant high level ROS production were verified in these oxygenated tumor cells. It is worth noting that extracellular ROS generation may also cause the small-molecule oxidants to induce cell apoptosis and inhibit cell proliferation, in the case of Ce6 molecules that are present in the ECM without intracellular internalization.65
In another aspect, the SH-laden agarose-based hydrogel was responsive to NIR light to produce local hyperthermia, which not only initiated local PTT but also triggered effective Ce6 release. Simultaneously, thermal-induced sol–gel phase transition also activated the rapid hydrolysis of hydrogel, which facilitated the introduction of interstitial fluid into the gel matrix to initiate oxygen generation. Thanks to the high controllability and good tumor-site retention of such smart hydrogel, systemic toxicity and local inflammation can be significantly prevented after administration as evidenced by comprehensive histopathological analysis. These results suggest a good opportunity of developing agarose-based hydrogel platforms for highly effective tumor treatments, which respond to both endogenous and exogenous factors.
Conclusion
In summary, an injectable agarose-based hydrogel was successfully prepared for enhanced photo-induced tumor therapy through the effective relief of hypoxia. Such a hybrid hydrogel exhibited good biocompatibility, high performance in photothermal conversion and light-triggered cargo release behavior. On account of the hydrolysis of the hydrogel under local hyperthermia, the incorporated MnO2 can effectively ameliorate hypoxia through catalytic decomposition of H2O2, which is produced in a high amount in the TME. Not only an increase of cellular internalization of Ce6 and elevation of the local O2 level, but also an efficacious PTT effect were achieved under NIR activation in vitro. Subsequently, enhanced PDT was achieved under 660 nm laser irradiation owing to the improvement of local oxygenation. Remarkably, merely single intratumoral injection of the hybrid hydrogel can produce a significant tumor inhibition effect under light activation in vivo, without causing a pathological lesion in the primary organs. Overall, the injectable SH/MnO2/Ce6 incorporated agarose hydrogel offers new opportunities for tumor microenvironment modulation, and inspires the development of novel hydrogel-based strategies towards clinical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by the Technology Innovation and Application Demonstration Grant of Chongqing (cstc2018jscx-msybX0078) and the National Natural Science Foundation of China (51703186, 31671037).Notes and references
- L. A. Torre, R. L. Siegel, E. M. Ward and A. Jemal, Cancer Epidemiol., Biomarkers Prev., 2016, 25, 16–27 CrossRef.
- P. Vineis and C. P. Wild, Lancet, 2014, 383, 549–557 CrossRef.
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre and A. Jemal, Ca-Cancer J. Clin., 2018, 68, 394–424 CrossRef.
- M. Arruebo, N. Vilaboa, B. Sáez-Gutierrez, J. Lambea, A. Tres, M. Valladares and Á. González-Fernández, Cancers, 2011, 3, 3279–3330 CrossRef CAS.
- H. Maeda and M. Khatami, Clin. Transl. Med., 2018, 7, 11 CrossRef.
- A. J. Bishop, G. K. Zagars, E. G. Demicco, W.-L. Wang, B. W. Feig and B. A. Guadagnolo, Am. J. Clin. Oncol., 2018, 41, 81–85 CrossRef.
- C. Y. Huang, D. T. Ju, C. F. Chang, P. M. Reddy and B. K. Velmurugan, Biomedicine, 2017, 7, 23 CrossRef.
- J. Zekri and S. Imtiaz, Radiother. Oncol., 2015, 114, 413–414 CrossRef.
- Y. H. Bae and K. Park, J. Controlled Release, 2011, 153, 198 CrossRef CAS.
- B. Bahrami, M. Hojjat-Farsangi, H. Mohammadi, E. Anvari, G. Ghalamfarsa, M. Yousefi and F. Jadidi-Niaragh, Immunol. Lett., 2017, 190, 64–83 CrossRef CAS.
- H. Wang, Q. Huang, H. Chang, J. Xiao and Y. Cheng, Biomater. Sci., 2016, 4, 375–390 RSC.
- Q. Wang, J. M. Li, H. Yu, K. Deng, W. Zhou, C. X. Wang, Y. Zhang, K. H. Li, R. X. Zhuo and S. W. Huang, Biomater. Sci., 2018, 6, 3096–3107 RSC.
- L. Sercombe, T. Veerati, F. Moheimani, S. Y. Wu, A. K. Sood and S. Hua, Front. Pharmacol., 2015, 6, 286 Search PubMed.
- G. A. Hughes, Nanomedicine, 2005, 1, 22–30 CrossRef CAS.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS.
- R. Dong, Y. Pang, Y. Su and X. Zhu, Biomater. Sci., 2015, 3, 937–954 RSC.
- C. Wang, X. Wang, K. Dong, J. Luo, Q. Zhang and Y. Cheng, Biomaterials, 2016, 104, 129–137 CrossRef CAS.
- C. Wang, D. Wang, T. Dai, P. Xu, P. Wu, Y. Zou, P. Yang, J. Hu, Y. Li and Y. Cheng, Adv. Funct. Mater., 2018, 28, 1802127 CrossRef.
- J. Hu, Y. Chen, Y. Li, Z. Zhou and Y. Cheng, Biomaterials, 2017, 112, 133–140 CrossRef CAS PubMed.
- X. Wang, C. Wang, X. Wang, Y. Wang, Q. Zhang and Y. Cheng, Chem. Mater., 2017, 29, 1370–1376 CrossRef CAS.
- W. Hu, Z. Wang, Y. Xiao, S. Zhang and J. Wang, Biomater. Sci., 2019, 7, 843–855 RSC.
- H. Cao, Y. Duan, Q. Lin, Y. Yang, Z. Gong, Y. Zhong, X. Chen and Z. Shao, Biomater. Sci., 2019, 7, 2975–2985 RSC.
- C. Delattre, T. A. Fenoradosoa and P. Michaud, Braz. Arch. Biol. Technol., 2011, 54, 1075–1092 CrossRef CAS.
- Q. Xu, S. Zhao, L. Deng, J. Ouyang, M. Wen, K. Zeng, L. Zhang, W. Chen and Y.-N. Liu, Chem. Commun., 2019, 55, 9471–9474 RSC.
- M. Qiu, D. Wang, W. Liang, L. Liu, Y. Zhang, X. Chen, D. K. Sang, C. Xing, Z. Li and B. Dong, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 501–506 CrossRef CAS.
- M. Hou, R. Yang, L. Zhang, L. Zhang, G. Liu, Z. Xu, Y. Kang and P. Xue, ACS Biomater. Sci. Eng., 2018, 4, 4266–4277 CrossRef CAS.
- R. Yang, M. Hou, Y. Gao, L. Zhang, Z. Xu, Y. Kang and P. Xue, Nanoscale, 2019, 11, 5717–5731 RSC.
- X. Wang, Y. Ma, H. Chen, X. Wu, H. Qian, X. Yang and Z. Zha, Colloids Surf., B, 2017, 152, 449–458 CrossRef CAS.
- X. Wang, Z. Miao, Y. Ma, H. Chen, H. Qian and Z. Zha, Nanoscale, 2017, 9, 14512–14519 RSC.
- P. Xue, R. Yang, L. Sun, Q. Li, L. Zhang, Z. Xu and Y. Kang, Nano-Micro Lett., 2018, 10, 74 CrossRef CAS.
- S. Peng, Y. He, M. Er, Y. Sheng, Y. Gu and H. Chen, Biomater. Sci., 2017, 5, 475–484 RSC.
- H. Chen, Y. Ma, X. Wang and Z. Zha, J. Mater. Chem. B, 2017, 5, 7051–7058 RSC.
- H. Chen, Y. Ma, X. Wang, X. Wu and Z. Zha, RSC Adv., 2017, 7, 248–255 RSC.
- J. E. Sun, B. Stewart, A. Litan, S. J. Lee, J. P. Schneider, S. A. Langhans and D. J. Pochan, Biomater. Sci., 2016, 4, 839–848 RSC.
- F. Raza, Y. Zhu, L. Chen, X. You, J. Zhang, A. Khan, M. W. Khan, M. Hasnat, H. Zafar and J. Wu, Biomater. Sci., 2019, 7, 2023–2036 RSC.
- X. Wang, F. Li, X. Yan, Y. Ma, Z. Miao, L. Dong, H. Chen, Y. Lu and Z. Zha, ACS Appl. Mater. Interfaces, 2017, 9, 41782–41793 CrossRef CAS.
- Z. H. Miao, K. Li, P. Y. Liu, Z. Li, H. Yang, Q. Zhao, M. Chang, Q. Yang, L. Zhen and C. Y. Xu, Adv. Healthcare Mater., 2018, 7, 1701202 CrossRef.
- J. J. Hu, Y. J. Cheng and X. Z. Zhang, Nanoscale, 2018, 10, 22657–22672 RSC.
- Y. Kihara, M. Tanaka, S. Gumiri, T. Hosokawa, S. Tanaka, T. Saito and M. Kurasaki, Environ. Toxicol., 2014, 29, 916–925 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS.
- F. Yuan, J. L. Li, H. Cheng, X. Zeng and X. Z. Zhang, Biomater. Sci., 2018, 6, 96–100 RSC.
- J. Deng, F. Liu, L. Wang, Y. An, M. Gao, Z. Wang and Y. Zhao, Biomater. Sci., 2019, 7, 429–441 RSC.
- R. Kumar, E.-J. Kim, J. Han, H. Lee, W. S. Shin, H. M. Kim, S. Bhuniya, J. S. Kim and K. S. Hong, Biomaterials, 2016, 104, 119–128 CrossRef CAS.
- S. V. Karakashev and M. J. Reginato, Cancer Manage. Res., 2015, 7, 253 CAS.
- J. Dang, H. He, D. Chen and L. Yin, Biomater. Sci., 2017, 5, 1500–1511 RSC.
- X. Li, N. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522–11531 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, J. Li, Z.-H. Peng, Y. Sheinin, J. Zhou and D. Oupický, ACS Nano, 2017, 11, 2227–2238 CrossRef CAS PubMed.
- Q. Chen, L. Feng, J. Liu, W. Zhu, Z. Dong, Y. Wu and Z. Liu, Adv. Mater., 2016, 28, 7129–7136 CrossRef CAS.
- W. Zhang, S. Li, X. Liu, C. Yang, N. Hu, L. Dou, B. Zhao, Q. Zhang, Y. Suo and J. Wang, Adv. Funct. Mater., 2018, 28, 1706375 CrossRef.
- Z. Ma, X. Jia, J. Bai, Y. Ruan, C. Wang, J. Li, M. Zhang and X. Jiang, Adv. Funct. Mater., 2017, 27, 1604258 CrossRef.
- J. Zhu and J. He, ACS Appl. Mater. Interfaces, 2012, 4, 1770–1776 CrossRef CAS.
- H. Chen, J. He, C. Zhang and H. He, J. Phys. Chem. C, 2007, 111, 18033–18038 CrossRef CAS.
- D. He, X. He, K. Wang, X. Yang, X. Yang, Z. Zou and X. Li, Chem. Commun., 2015, 51, 776–779 RSC.
- C. Yuan, B. Gao, L. Su and X. Zhang, J. Colloid Interface Sci., 2008, 322, 545–550 CrossRef CAS.
- Q. Y. Cai, J. Li, J. Ge, L. Zhang, Y. L. Hu, Z. H. Li and L. B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef CAS.
- L. Sun, Q. Li, M. Hou, Y. Gao, R. Yang, L. Zhang, Z. Xu, Y. Kang and P. Xue, Biomater. Sci., 2018, 6, 2881–2895 RSC.
- T. Singh, T. J. Trivedi and A. Kumar, Green Chem., 2010, 12, 1029–1035 RSC.
- M. Bilal, T. Rasheed, Y. Zhao and H. M. Iqbal, Int. J. Biol. Macromol., 2019, 124, 742–749 CrossRef CAS.
- P. de Andrade Mello, S. Bian, L. E. B. Savio, H. Zhang, J. Zhang, W. Junger, M. R. Wink, G. Lenz, A. Buffon and Y. Wu, Oncotarget, 2017, 8, 67254 Search PubMed.
- N. Lu, P. Huang, W. Fan, Z. Wang, Y. Liu, S. Wang, G. Zhang, J. Hu, W. Liu and G. Niu, Biomaterials, 2017, 126, 39–48 CrossRef CAS.
- Y. S. Chen, S. J. Yoon, W. Frey, M. Dockery and S. Emelianov, Nat. Commun., 2017, 8, 15782 CrossRef CAS PubMed.
- D. Jaque, L. M. Maestro, B. Del Rosal, P. Haro-Gonzalez, A. Benayas, J. Plaza, E. M. Rodriguez and J. G. Sole, Nanoscale, 2014, 6, 9494–9530 RSC.
- J. Pouysségur, F. Dayan and N. M. Mazure, Nature, 2006, 441, 437 CrossRef.
- M. Gao, C. Liang, X. Song, Q. Chen, Q. Jin, C. Wang and Z. Liu, Adv. Mater., 2017, 29, 1701429 CrossRef.
- B. Kalyanaraman, G. Cheng, M. Hardy, O. Ouari, B. Bennett and J. Zielonka, Redox Biol., 2018, 15, 347–362 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9bm01472a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |