3D biofabrication of microfiber-laden minispheroids: a facile 3D cell co-culturing system†
Mingjun
Xie
abc,
Qing
Gao
abc,
Jingjiang
Qiu
abc,
Jianzhong
Fu
abc,
Zichen
Chen
abc and
Yong
He
 *abc
*abc
aState Key Laboratory of Fluid Power and Mechatronic Systems, School of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: yongqin@zju.edu.cn
bKey Laboratory of 3D Printing Process and Equipment of Zhejiang Province, School of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China
cSchool of Mechanics and Safety Engineering, Zhengzhou University, Zhengzhou 450001, China
First published on 25th November 2019
Abstract
Hierarchical tissues composed of spheroid and fiber structures such as tumors, embryos and glomeruli widely exist in organisms. Methods have been developed to build spheroid and fiber structures, independently, as tissue models in vitro. However, it is still a challenge to print them simultaneously and integrated for effectively mimicking the complicated situations in vivo. Here, we propose a novel 3D cell co-culturing system, “microfiber-laden minispheroid”, applying two fluidic phenomena, namely the “rope coiling effect” and “electrohydrodynamics”, with a co-axial bioprinting nozzle and high voltage system. Gelatin methacryloyl (GelMA) hydrogels were extruded from the outer nozzle and separated by electrical attraction to form spheroids (∼1800 μm). GelMA mixed with sodium alginate was extruded from the inner nozzle to form fibers (∼180 μm) inside spheroids, whose morphology could be controlled by the ratio of inner and outer nozzle extruding flow rates. We analyzed the fabrication process and the material system in detail, verifying the fabrication feasibility and suitable microenvironment. The encapsulated cells possessed high viabilities. Importantly, the actin of human umbilical vein endothelial cells tended to elongate towards the co-cultured tumor cells, in contrast to the HUVECs cultured alone. We believe that “microfiber-laden minispheroids” could be a potential system for 3D cell co-culturing research in the future.
The spheroid is a common structure in organisms, such as embryos, glomeruli, and tumors. With the development of biofabrication technology,1,2 lots of approaches to establish spheroid structures as tissue models in vitro have been proposed.3–7 Besides the spheroid, the fiber is another significant structure in vivo, such as those found in lymph-vessels, muscle fibers and blood vessels,8 whose shapes can be more diversified. Taking blood vessels as an example, not only do they comprise straight and curved shapes, but also helical morphology, such as for the cochlear spiral modiolar artery, endometrial spiral arterioles and intestinal villi spiral arterioles.9,10 Therefore, a series of approaches to produce cell-laden microfibers in vitro have been developed.11–15 These microfibers can effectively mimic the morphological and biological characters of the fiber-shaped structures in vivo.
However, in actual organisms, lots of tissues are composed of both spheroid and fiber structures rather than a single type of them. To mimic the cell interactions in these tissues in vivo, building hierarchical tissue models in vitro with different structure types is urgently needed, which has a far-reaching significance in the field of biomedical research. Taking tumor tissues for instance, in actual organisms, when tumors (spheroid structures) grow up to the diameter of 1 mm, the tumor cells tend to release specific biotic factors to promote the endothelial cells to spread towards them and to form new vessels for providing more nutrients and oxygen. In fact, there are usually blood vessels (fiber structures) inside the tumor tissues for more material exchange. In recent years, antitumor drugs such as traditional paclitaxel and other therapeutic drugs have been gradually replaced by anti-angiogenesis drugs. From this aspect, the structure of fibers encapsulated in a sphere has practical biological significance and is expected to become a new drug screening system in the future to study advanced antitumor drugs.
Obviously, most of the existing models with a single type of structure are too simple to carry out further research with. Thus, although a series of approaches to build spheroids or fibers independently have been proposed, it is still a challenge to realize the simultaneous formation of spheroids and encapsulated fibers inside. This kind of structure could provide more realistic simulation of some organs or tissues in actual organisms and are expected to become a hierarchical structure integration as a 3D cellular co-culturing model. Currently, the fabrication of hierarchical models mainly depends on two kinds of methods. In the first method, a two-phase fabrication process is mostly designed to build a single type of structure, namely co-axial fibers or core–shell spheres.16–20 In the second method, in terms of more structural types, taking microsphere-laden stereolithography-based fabrication as an example,21 researchers have to follow two steps, namely fabricating the encapsulated microspheres in advance and then mixing them in the outer phase liquid for stereolithography-based fabrication. Hence, it strikes us that we can print these two different types of structures simultaneously, forming a brand-new tissue co-culturing system for effectively mimicking the complicated situations in vivo.
Besides a feasible fabrication method, a suitable material system applied for the co-culturing model is also considered as a significant part. The established 3D microenvironment in vitro must be similar to the in vivo conditions. Hydrogels have become a promising material for the establishment of tissue models and have been widely used in a series of biofabrication methods.22–25 Recently, gelatin methacryloyl (GelMA), which is synthesized from the mammalian gelatin degraded from collagen,26,27 has been playing a significant role in a series of biomedical applications, with its capabilities of achieving cellular functionalization and rapid crosslinking.28–32 Thus, this material is thought to have great potential to be applied for establishing co-culturing structures.
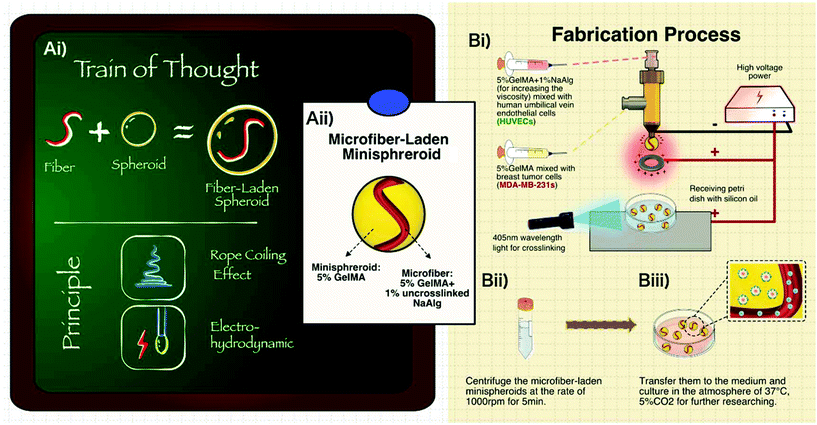
In this paper, integrating the fiber and spheroid structures (Fig. 1Ai), a pioneering 3D cell co-culturing system, namely “microfiber-laden minispheroids”, was invented. We proposed a one-step manufacturing method that combined spherical structures with fiber structures. Inspired by a famous fluidic phenomenon, the “rope coiling effect”,33–37 which occurred under the effects of gravity, inertia force and friction deriving from the inner and outer flow rate difference, the microfibers and minispheroids could be fabricated simultaneously with a co-axial biofabrication nozzle. The pure GelMA was extruded from the outer nozzle and then formed spheroids (∼1800 μm). The GelMA mixed with sodium alginate was extruded from the inner nozzle and then formed fibers (∼180 μm), as shown in Fig. 1Aii. It should be noted that the sodium alginate was not crosslinked during the fabrication process. It was just utilized to increase the liquid viscosity so that the “rope coiling effect” could occur and the fiber structure inside could be maintained when the droplets dripped into the oil. The fiber size was controlled by the ratio of two-phase flow rates. Besides, in our previous work,38 we have developed a promising electro-assisted method for rapid and scalable fabrication of GelMA microspheres with a single flow-path nozzle. According to the electrohydrodynamic (EHD) principle,39–43 electric force could provide additional force to separate droplets. Thus, the co-axial nozzle in this work was exposed to a similar high voltage electric field to adjust the general size of the structure. We anticipate that this brand-new structure and the pioneering fabrication method will play an important role in 3D cell co-culturing and can contribute to advance the development of more biomedical applications.
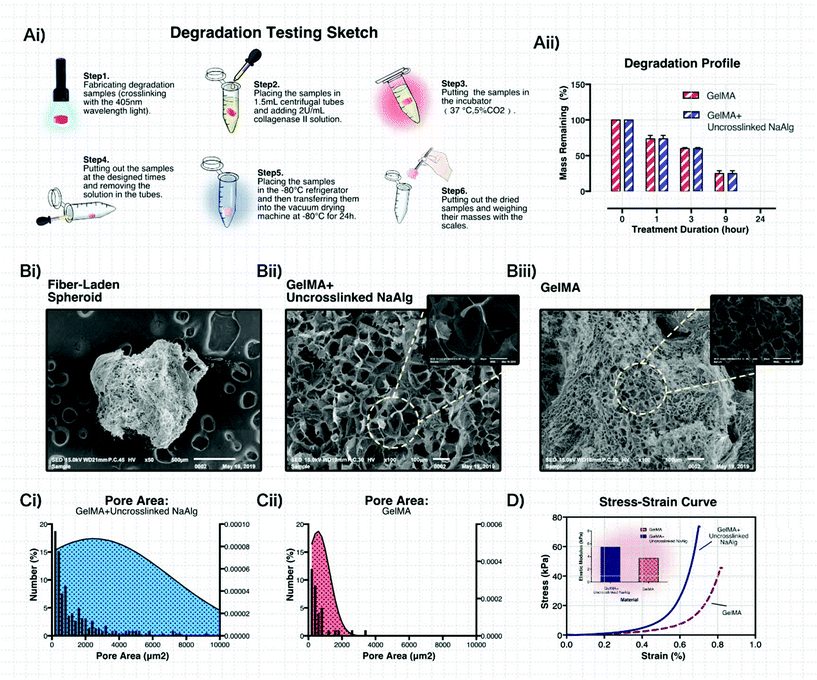
The sketch of the fabrication devices and process is shown in Fig. 1B. A co-axial biofabrication nozzle was connected to the negative pole of the high voltage system and a metal ring was connected to the positive pole, forming a high voltage electric field. The droplets from the nozzle could be filled with negative electrics. To avoid the backward movement of the droplets because of the attraction of the positive metal ring after going through it, a metal plate connected to the positive pole was placed below the metal ring to make the droplets continually drip down. The droplets were obtained using silicon oil and crosslinked by 405 nm wavelength light. After crosslinking, the structures were transferred to centrifugal tubes and then centrifuged at the rate of 1000 rpm for 5 min. Finally, the structures were cultured in a medium for carrying out further research.
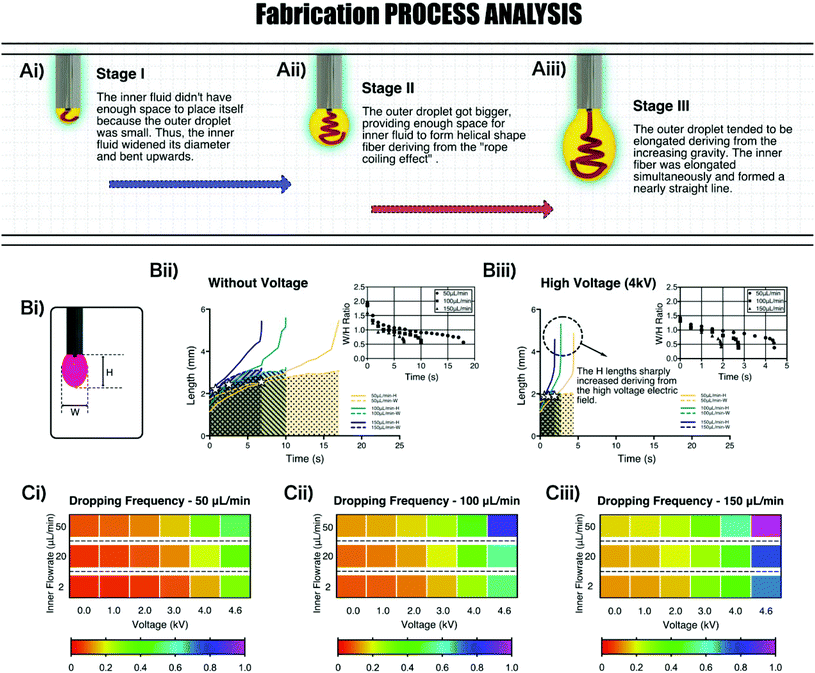
To deeply analyze the flow states of the two-phase fluids, the basic fabrication process without voltage was observed with a high-speed camera. The sketches of three crucial stages are shown in Fig. 2Ai–iii. At stage I, the outer liquid was too little to provide enough space for placing the inner liquid. The inner fluid tended to widen the diameter and bend upwards inside the droplet to make more space for itself ( for additional discussion, see the ESI†). At stage II, the outer droplet became bigger, which could offer much more space for the inner liquid. Because of the flow rate difference between the inner and outer liquid, a phenomenon in fluid mechanics, “rope coiling effect”, occurred here. The inner fluid tended to rotate and form a helical shape fiber inside the outer droplet. At stage III, the outer droplet became much bigger. The increasing gravity made it elongated. Thus, at the end of the fabrication process, the inner fluid tended to be straightened simultaneously, forming a nearly straight fiber. Remarkably, under the conditions of adding high voltage, the dropping cycle was shorter and the elongation effect of the outer droplet was stronger. Thus, the duration proportions of stage I and stage III could be longer in contrast to the situations without voltage (Fig. S2, ESI†).
According to the above research studies, we found that either the width or height of the outer droplet around the nozzle was the defining parameter in the fabrication process, which could influence the shape of the inner fiber. With the help of a high-speed camera, the formation process with different fabrication parameters was recorded. The width and height data were obtained using ImageJ software (Fig. 2Bii–iii). The width and height of the droplet were both monotonically increased with time going whether or not the high voltage was applied. At first, the width of the droplet was generally larger than the height. Then, the height increased at a higher rate than the width, resulting in an increase of the ratio of width/height (W/H). Besides, the inner flow rate had a significant influence on the ratio. The higher the inner flow rate was, the faster the width and height increased. It was because the inner fluid could provide additional gravity to the dropping system, which shortened the dropping cycle. What's more, comparing the data of the no-voltage group and high-voltage group, we could see that increasing the electric voltage could further enhance the rate of increase of the W/H ratio. The initial W/H values in the high-voltage group were lower than the ones in the no-voltage group. These reasons made the fibers in the high-voltage group straighter and with less helical structures than the ones the in no-voltage group.
Dropping frequency was another crucial dependent variable in that it decided the general size of the structure and the fabrication efficiency. Here, the dropping frequency was analyzed with the recording videos and summarized in Fig. 2Ci–iii. The frequency data were indicated by the colors of the rectangles. From the graphs, we could conclude that a higher outer flow rate caused a higher dropping frequency. Furthermore, when the voltage was provided to the fabrication system, with the increase of voltage, the frequency also increased. Besides, the inner fluid could also provide additional gravity for the droplet system, thus a higher inner flow rate resulted in a higher dropping frequency.
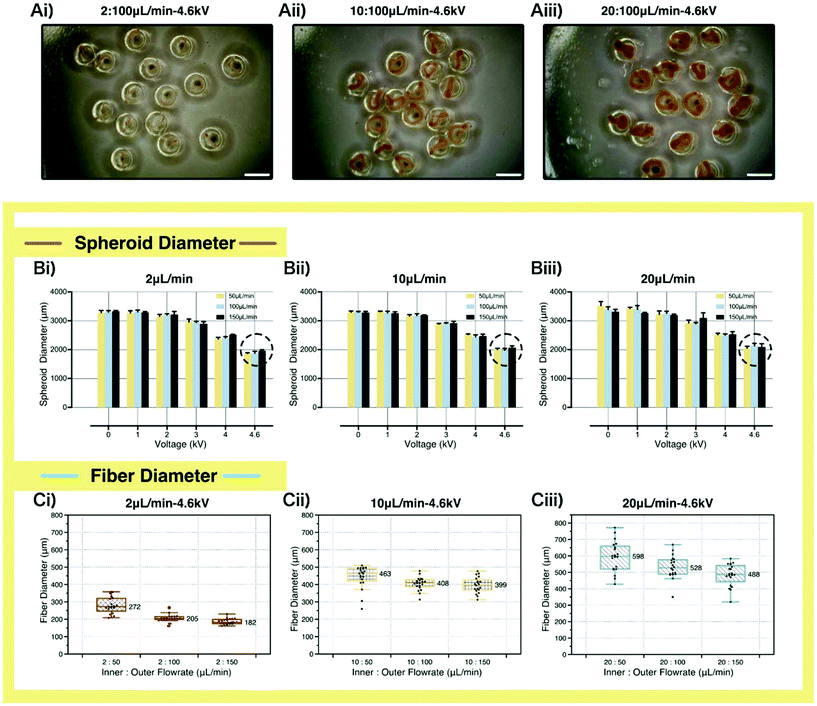
Fabrication parameters could influence the formation of fiber-laden spheroids, as shown in Fig. 3Ai–iii. To analyze the effects of the fabrication parameters, namely voltages, inner and outer flow rates, on the sizes of the fiber and spheroid, we fabricated a series of structures and recorded their diameters. The results are shown in Fig. 3Bi–Ciii. We could find that the spheroid diameter decreased with the increase of the applied voltage. Besides, when a higher voltage was applied (4.6 kV), the higher inner flow rate led to a higher spheroid size, in that the inner fiber occupied extra space inside the spheroid. In terms of fiber size, we summarized the data in the box plots. As we can see, when the outer flow rates were set the same, thicker fibers were fabricated with higher inner flow rates. And when the inner flow rates were set the same, thinner fibers were fabricated with higher outer flow rates resulting from the elongation effect of the outer droplet on the inner fibers. What's more, higher inner flow rates led to a wider fiber diameter distribution.
From the examinations and discussions above, the fabrication approach for microfiber-laden minispheroids was verified to be feasible. In addition, in this strategy, pure GelMA and GelMA mixed with uncrosslinked sodium alginate were applied to build the microenvironment for the encapsulated cells. Considering that the material system would have a great influence on cell growth and functionalization, the material properties were examined in detail next.
Firstly, the degradation profiles of 5% GelMA and 5% GelMA mixed with 1% uncrosslinked sodium alginate were examined here. The sketch of the degradation experiment in our work is displayed in Fig. 4Ai. The degradation samples were immersed in a DPBS solution containing 2 U ml−1 of collagenase II.44,45 The test results (Fig. 4Aii) showed that the samples made with 5% GelMA and 5% GelMA mixed with 1% uncrosslinked sodium alginate were completely degraded within 24 h, suggesting that the materials we applied here had the ability to respond to a biological environment (Fig. S4, ESI†).
With optical microscopy, many pores on the surface of the crosslinked 5% GelMA mixed with 1% uncrosslinked sodium alginate could be found (Fig. S3, ESI†). For further material analysis, scanning electron microscopy (SEM) was used to examine the morphology of the material system. The SEM images shown in Fig. 4Bi–iii reveal that numerous micropores were formed after the stereolithography process, indicating that the microfiber-laden minispheroids could achieve the material exchange between the inner and outer environment of the structure and provide enough space for cell survival and spreading. The areas of the inner pores were analyzed using ImageJ software (Fig. 4Ci and ii). Importantly, we could find that the pores established using 5% GelMA mixed with 1% uncrosslinked sodium alginate were much larger than the ones established using pure 5% GelMA. It was because the alginate ions occupied some space among the GelMA molecules. When the materials were exposed to 405 nm light, GelMA molecules provided space for alginate ions and formed larger pores.
The mechanical properties of the materials were considered crucial for the encapsulated cell growth. As shown in Fig. 4D, the two applied materials showed promising stiffness. The elastic modulus of the pure 5% GelMA was 3.731 kPa and the modulus of the 5% GelMA mixed with 1% uncrosslinked alginate sodium was 5.451 kPa. This phenomenon could be explained according to the SEM morphology and pore area distribution of the material. The larger hydrogel networks made the crosslinked materials have higher stiffness.
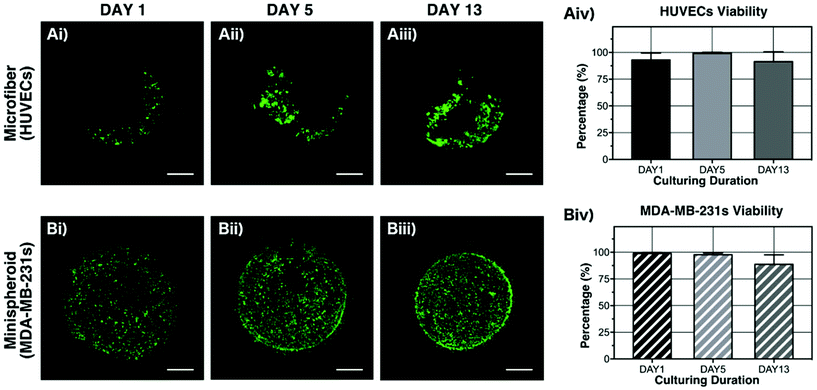
In terms of encapsulated cells, the cells experienced high voltage electric force and shear force in this fabrication strategy. Thus, the biocompatibility of the proposed structure is important to be examined. Here, HUVECs and MDA-MB-231s were respectively encapsulated in the fiber and spheroid, independently, with the proposed method. Firstly, the viabilities of the HUVECs in the fiber and the MDA-MB-231s in the spheroid were tested with the LIVE/DEAD reagent on the 1st, 5th, and 13th day of culturing. The confocal microscopy images are shown in Fig. 5Ai–iii and Bi–iii. The numbers of live and dead cells were analyzed using the ImageJ software. As shown in Fig. 5Aiv and Biv, the viabilities of the HUVECs and MDA-MB-231s remained above 88%, which indicated that this fabrication approach and the applied material system could keep the cellular activity.
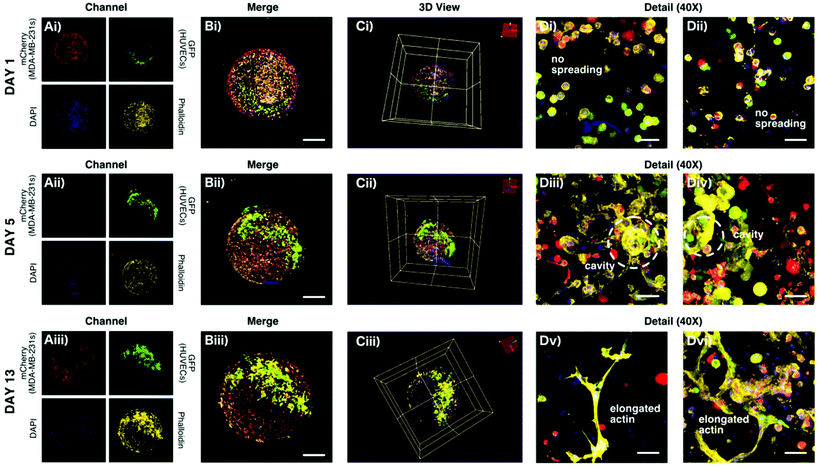
To examine the co-culturing effect of the proposed microfiber-laden minispheroid, transfected HUVECs with GFP were encapsulated in the microfibers and transfected MDA-MB-231s with mCherry were encapsulated in the minispheroids simultaneously. The models were cultured for 13 days. The cellular morphologies were captured on the 1st, 5th and 13th day. The nucleus and the actin of the cells were stained with DAPI and phalloidin, respectively, as shown in Fig. 6Ai–Ciii. From these general figures, we could find a clear boundary between the fibers and the spheroids. The details of the encapsulated cells are given in Fig. 6Di–vi. On the first day, the HUVECs and MDA-MB-231s maintained a globular shape and didn't spread. On the fifth day, the two kinds of cells showed obvious spreading. Remarkably, the HUVECs encapsulated in the microfibers tended to form a cavity. It could be easily explained with the SEM morphology of the material. There were lots of pores with much larger sizes than the cells. Thus, the HUVECs could easily attach to the pore walls and form cavity-shaped structures. This finding indicated that the uncrosslinked sodium alginate didn't affect the spreading ability of the encapsulated cells. In contrast, it provided wider space for the encapsulated cells. On the thirteenth day, under the influence of the tumor cells in the co-culturing system, the actin on the cell membrane of the HUVECs distributed widely inside the hydrogel network and spread towards the outer tumor cells, whereas the HUVECs cultured without tumor cells in the microfiber-laden minispheroids kept spreading in the cavity and didn't show obvious elongation outwards (Fig. S5, ESI†). All of these results showed that this co-culturing system showed very similar morphology or structure to the tissues in organisms and could be more suitable for mimicking the complex situations in vivo.
In summary, combining the widely-used fiber structures and spheroid structures in the biomedical field, in this paper, we proposed a creative structure, “microfiber-laden minispheroid” to be applied as a brand-new co-culturing system. The challenge of simultaneously fabricating different types of structures was solved. The fabrication system mainly consisted of a co-axial biofabrication nozzle and high voltage system. Two famous fluidic phenomena, namely the “rope coiling effect” and “electrohydrodynamics”, were utilized to realize the simultaneous formation of structures and size control. The GelMA hydrogel was chosen as the basic material of the co-culturing system. Pure GelMA flowed from the outer nozzle to form the spheroid structure. GelMA mixed with sodium alginate (for increasing viscosity) flowed from the inner nozzle to form the fiber structure inside the spheroid. The fabrication process and the parameter effect on the sizes of the fibers and spheroids were analyzed in detail, confirming the fabrication feasibility of this strategy. The material system was found to have promising SEM morphology, degradation profile, pore area distribution and stiffness. Remarkably, GelMA mixed with uncrosslinked alginate sodium showed much larger pores than pure GelMA, providing more space for material exchange and cell growth. Furthermore, we proved that the encapsulated HUVECs and MDA-MB-231s maintained high viabilities. Importantly, the co-culturing results of transfected HUVECs (GFP) and MDA-MB-231s (mCherry) showed obvious interactions between the two kinds of cells. Under the effect of the tumor cells, the actin of the HUVECs gradually elongated towards the tumor cells after thirteen-day culturing, whereas the HUVEC culture alone didn't show the same phenomenon. We anticipate that the pioneering “microfiber-laden minispheroid” has very similar morphology or structure to the tissues in organisms and could be more suitable for mimicking the complex situations in vivo. This fabrication method will play an important role in 3D cell co-culturing research and can contribute to advance the development of more biomedical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by the National Key Research and Development Program of China (2018YFA0703000), the National Nature Science Foundation of China (No. U1609207, 81827804), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No. 51821093).References
- S. Ahadian and A. Khademhosseini, Bio-Des. Manuf., 2018, 1, 157 CrossRef PubMed.
- H. Jian, M. Wang, S. Wang, A. Wang and S. Bai, Bio-Des. Manuf., 2018, 1, 45 CrossRef CAS.
- E. Fennema, N. Rivron, J. Rouwkema, C. van Blitterswijk and J. de Boer, Trends Biotechnol., 2013, 31, 108 CrossRef CAS PubMed.
- J. Fukuda, A. Khademhosseini, Y. Yeo, X. Yang, J. Yeh, G. Eng, J. Blumling, C.-F. Wang, D. S. Kohane and R. Langer, Biomaterials, 2006, 27, 5259 CrossRef CAS PubMed.
- M. W. Laschke and M. D. Menger, Trends Biotechnol., 2017, 35, 133 CrossRef CAS PubMed.
- J. Liao, B. Wang, Y. Huang, Y. Qu, J. Peng and Z. Qian, ACS Omega, 2017, 2, 443 CrossRef CAS PubMed.
- J. M. Santos, S. P. Camões, E. Filipe, M. Cipriano, R. N. Barcia, M. Filipe, M. Teixeira, S. Simões, M. Gaspar, D. Mosqueira, D. S. Nascimento, P. Pinto-do-Ó, P. Cruz, H. Cruz, M. Castro and J. P. Miranda, Stem Cell Res. Ther., 2015, 6, 90 CrossRef PubMed.
- D. H. Yoon, K. Kobayashi, D. Tanaka, T. Sekiguchi and S. Shoji, Lab Chip, 2017, 17, 1481 RSC.
- F. De Wolf, C. De Wolf-Peeters and I. Brosens, Am. J. Obstet. Gynecol., 1973, 117, 833 CrossRef CAS PubMed.
- R. Pijnenborg, L. Vercruysse and M. Hanssens, Placenta, 2006, 27, 939 CrossRef CAS PubMed.
- A. Y. Hsiao, T. Okitsu, H. Onoe, M. Kiyosawa, H. Teramae, S. Iwanaga, T. Kazama, T. Matsumoto and S. Takeuchi, PLoS One, 2015, 10, e0119010 CrossRef PubMed.
- Z.-J. Meng, W. Wang, R. Xie, X.-J. Ju, Z. Liu and L.-Y. Chu, Lab Chip, 2016, 16, 2673 RSC.
- L. Peng, Y. Liu, J. Gong, K. Zhang and J. Ma, RSC Adv., 2017, 7, 19243 RSC.
- M. Sugimoto, Y. Kitagawa, M. Yamada, Y. Yajima, R. Utoh and M. Seki, Lab Chip, 2018, 18, 1378 RSC.
- M. Yamada, S. Sugaya, Y. Naganuma and M. Seki, Soft Matter, 2012, 8, 3122 RSC.
- Q. Chen, S. Utech, D. Chen, R. Prodanovic, J.-M. Lin and D. A. Weitz, Lab Chip, 2016, 16, 1346 RSC.
- F. Fu, L. Shang, F. Zheng, Z. Chen, H. Wang, J. Wang, Z. Gu and Y. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 13840 CrossRef CAS PubMed.
- L. Shao, Q. Gao, C. Xie, J. Fu, M. Xiang and Y. He, Adv. Healthcare Mater., 2019, 8, 1900014 CrossRef PubMed.
- L. Shao, Q. Gao, H. Zhao, C. Xie, J. Fu, Z. Liu, M. Xiang and Y. He, Small, 2018, 14, 1802187 CrossRef PubMed.
- L. Yu, C. Ni, S. M. Grist, C. Bayly and K. C. Cheung, Biomed. Microdevices, 2015, 17, 33 CrossRef PubMed.
- W. Zhu, H. Cui, B. Boualam, F. Masood, E. Flynn, R. D. Rao, Z.-Y. Zhang and L. G. Zhang, Nanotechnology, 2018, 29, 185101 CrossRef PubMed.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105 CrossRef CAS PubMed.
- R. S. Ashton, A. Banerjee, S. Punyani, D. V. Schaffer and R. S. Kane, Biomaterials, 2007, 28, 5518 CrossRef CAS PubMed.
- T. Billiet, M. Vandenhaute, J. Schelfhout, S. Van Vlierberghe and P. Dubruel, Biomaterials, 2012, 33, 6020 CrossRef CAS PubMed.
- J. Saroia, W. Yanen, Q. Wei, K. Zhang, T. Lu and B. Zhang, Bio-Des. Manuf., 2018, 1, 265 CrossRef CAS.
- B. J. Rose, S. Pacelli, J. A. Haj, S. H. Dua, A. Hopkinson, J. L. White and R. F. Rose, Materials, 2014, 7, 3106–3135 CrossRef PubMed.
- G. Ying, N. Jiang, C. Yu and Y. S. Zhang, Bio-Des. Manuf., 2018, 1, 215 CrossRef CAS.
- Q. Gao, X. Niu, L. Shao, L. Zhou, Z. Lin, A. Sun, J. Fu, Z. Chen, J. Hu, Y. Liu and Y. He, Biofabrication, 2019, 11, 035006 CrossRef PubMed.
- P. Hassanzadeh, M. Kazemzadeh-Narbat, R. Rosenzweig, X. Zhang, A. Khademhosseini, N. Annabi and M. Rolandi, J. Mater. Chem. B, 2016, 4, 2539 RSC.
- C. McBeth, J. Lauer, M. Ottersbach, J. Campbell, A. Sharon and A. F. Sauer-Budge, Biofabrication, 2017, 9, 015009 CrossRef PubMed.
- J. Nie, Q. Gao, Y. Wang, J. Zeng, H. Zhao, Y. Sun, J. Shen, H. Ramezani, Z. Fu, Z. Liu, M. Xiang, J. Fu, P. Zhao, W. Chen and Y. He, Small, 2018, 14, 1802368 CrossRef PubMed.
- K. Yue, G. Trujillo-de Santiago, M. M. Alvarez, A. Tamayol, N. Annabi and A. Khademhosseini, Biomaterials, 2015, 73, 254 CrossRef CAS PubMed.
- M. Habibi, M. Maleki, R. Golestanian, N. M. Ribe and D. Bonn, Phys. Rev. E, 2006, 74, 066306 CrossRef PubMed.
- B. Jia, L. Yu, F. Fu, L. Li, J. Zhou and L. Zhang, RSC Adv., 2014, 4, 9112 RSC.
- S.-I. Nagahiro and Y. Hayakawa, Phys. Rev. E, 2008, 78, 025302 CrossRef PubMed.
- N. M. Ribe, M. Habibi and D. Bonn, Phys. Fluids, 2006, 18, 084102 CrossRef.
- N. M. Ribe, M. Habibi and D. Bonn, Annu. Rev. Fluid Mech., 2011, 44, 249 CrossRef.
- M. Xie, Q. Gao, H. Zhao, J. Nie, Z. Fu, H. Wang, L. Chen, L. Shao, J. Fu, Z. Chen and Y. He, Small, 2019, 15, 1804216 CrossRef PubMed.
- J. Q. Feng, Proc. R. Soc. London, Ser. A, 1999, 455, 2245 CrossRef CAS.
- E. D. Fylladitakis, M. P. Theodoridis and A. X. Moronis, IEEE Trans. Plasma Sci., 2014, 42, 358 Search PubMed.
- P. Goldberg-Oppenheimer and U. Steiner, Small, 2010, 6, 1248 CrossRef CAS PubMed.
- Y. Huang, N. Bu, Y. Duan, Y. Pan, H. Liu, Z. Yin and Y. Xiong, Nanoscale, 2013, 5, 12007 RSC.
- D. A. Saville, Annu. Rev. Fluid Mech., 1997, 29, 27 CrossRef.
- H. J. Yoon, S. R. Shin, J. M. Cha, S.-H. Lee, J.-H. Kim, J. T. Do, H. Song and H. Bae, PLoS One, 2016, 11, e0163902 CrossRef PubMed.
- X. Zhao, S. Liu, L. Yildirimer, H. Zhao, R. Ding, H. Wang, W. Cui and D. Weitz, Adv. Funct. Mater., 2016, 26, 2809 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9bm01189g |
| This journal is © The Royal Society of Chemistry 2020 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: