Silk based scaffolds with immunomodulatory capacity: anti-inflammatory effects of nicotinic acid†
Abdollah
Zakeri Siavashani
a,
Javad
Mohammadi
*b,
Katharina
Maniura-Weber
a,
Berna
Senturk
a,
Jhamak
Nourmohammadi
b,
Behnam
Sadeghi
c,
Lukas
Huber
d and
Markus
Rottmar
 *a
*a
aEmpa, Swiss Federal Laboratories for Materials Science and Technology, Biointerfaces, St.Gallen, Switzerland. E-mail: markus.rottmar@empa.ch
bFaculty of New Sciences and Technologies, University of Tehran, Tehran, Iran. E-mail: drjmohamadi@yahoo.com
cTranslational Cell therapy Research (TCR), Department of CLINTEC, Karolinska Institutet, Stockholm, Sweden
dEmpa, Swiss Federal Laboratories for Materials Science and Technology, Laboratory for Building Energy Materials and Components, Dübendorf, Switzerland
First published on 29th October 2019
Abstract
Implantation of temporary and permanent biomaterials in the body leads to a foreign body reaction (FBR), which may adversely affect tissue repair processes and functional integration of the biomaterial. However, modulation of the inflammatory response towards biomaterials can potentially enable a favorable healing response associated with functional tissue formation and tissue regeneration. In this work, incorporation of nicotinic acid in 3D silk scaffolds is explored as an immunomodulatory strategy for implantable biomaterials. Silk scaffolds were fabricated from dissolved Bombyx mori silk fibers by freeze-drying, resulting in silk scaffolds with high porosity (>94%), well-connected macropores, a high swelling degree (>550%) and resistance to in vitro degradation. Furthermore, drug-loaded scaffolds displayed a sustained drug release and excellent cytocompatibility could be observed with osteoblast-like MG63 cells. Cultivating M1-like macrophages on the scaffolds revealed that scaffolds loaded with nicotinic acid suppress gene expression of pro-inflammatory markers TNF-α, CXCL10 and CD197 as well as secretion of TNF-α in a concentration dependant manner. Hence, this study provides insights into the possible application of nicotinic acid in tissue engineering to control inflammatory responses towards biomaterials and potentially help minimizing FBR.
1. Introduction
The host immune response after implantation of biomaterials, known as foreign body reaction (FBR), can cause significant problems for patients through excessive inflammation and interference with the function of implanted biomaterials.1,2 As the FBR usually adversely affects tissue repair processes and functional integration of the biomaterial, controlling this immune reaction represents a universal challenge in the field of regenerative medicine and tissue engineered products.3–5The FBR is characterized by the presence of different immune cells including neutrophils, macrophages, dendritic cells, and lymphocytes at the implantation site and subsequent formation of granulation tissue, foreign body giant cells and a fibrous capsule around implanted biomaterials.2 Overactivation of neutrophils, persistent macrophage polarization into a pro-inflammatory phenotype, maturation of dendritic cells, and recruitment of lymphocytes is often associated with fibrotic response and scar tissue formation in the biomaterial integration process.6 Thus, modulation of inflammatory responses is necessary to enable a favorable healing result associated with functional tissue formation, reduction of tissue damage due to inflammation, minimizing chronic inflammation and improving tissue regeneration.7
The initial immune response to an implanted biomaterial determines whether the implant will be accepted or rejected as a foreign body by the immune system.8 Silk proteins are currently under investigation as potential biomaterials for tissue engineering applications for a variety of reasons, including their similarity to native extracellular matrix (ECM), their availability and possibility to easily process them into various material forms, as well as their ability to support the attachment of different cell types.9–11 However, there are some conflicting reports regarding a possible immunogenicity of silk proteins, which led researchers to separate sericin from fibroin through a procedure known as degumming prior to its use in biomedical applications.10,12,13 In fact, it has been observed that unlike fibroin and sericin alone, undegummed native silk products in the form of silk suture threads can induce a severe foreign body reaction or inflammation, delayed wound healing and induced immunoglobulin E (IgE) mediated allergy.12,14 Despite the great interest in separating fibroin and sericin before tissue engineering applications, degumming has been shown to negatively influence fibroin-based biomaterials after this thermo-chemical treatment by decreasing the molecular weight and mechanical properties of fibroin and sericin and also by interfering with the reproducibility of properties of produced samples.15–18 By avoiding the degumming process however, most of silk's components such as fibroin, sericin, seroin and P25/fibrohexamerin would be included within the fabricated scaffolds while the majority of small molecule impurities such as waxes, sugars and fats are expected to be removed during dialysis.19
Acute inflammation as the starting step of tissue repair can positively regulate the healing process and its symptoms normally disappear within a few days after injury. However, in the presence of a foreign body, its excessive reaction through high expression of pro-inflammatory cytokines and reactive oxygen species can be detrimental to the healing stages and is one of the underlying causes for subsequent failure of biomaterials.5,20 To address this issue, numerous studies have been conducted to modulate the biomaterial–immune system interactions through various strategies such as incorporation of anti-inflammatory molecules, which have resulted in significant improvements in the healing process due to decreasing levels of pro-inflammatory cytokines.1,21 Being crucial for tissue repair in general, modulation of the immune response has attracted great interest, also in the development of bone biomaterials.5 It is known that there is a direct crosstalk between the skeletal system and immune cells, which can shift tissue–biomaterial interaction to osteogenesis or osteolysis. Hence, a new generation of biomaterials with osteoimmunomodulatory capacities is rapidly emerging.22–24 Among them, local delivery of immunomodulatory agents is the most successful strategy for overcoming inflammation and fibrosis.7,25 To this end, anti-inflammatory cytokines and growth factors such as IL-10 and TGF-β have been immobilized within implanted biomaterials to control inflammatory responses.26,27 Also, common immunosuppressive drugs like calcineurin inhibitors, glucocorticoids and anti-TNF-α antibodies have been used to promote the functionality and tissue regeneration around the biomaterials.28–30 For instance, inhibiting inflammation by loading collagen-hydrogels with resveratrol improved bone and cartilage regeneration and repair,31 suppression of IFN-γ and TNF-α was able to enhance bone regeneration32 and incorporating resolvin D1 in chitosan 3D sponges showed a general decrease in pro-inflammatory cytokines.33 Regarding silk-based biomaterials, Kweon and colleagues have reported that 4-hexylresorcinol is able to inhibit FBGC formation in response to silk fibroin.10 In spite of the great therapeutic potential of these approaches, application of most immunosuppressive agents in tissue engineering is limited due to poor water solubility as well as low stability and short half-life of the bioactive component under physiological conditions, but also due to difficult handling, high cost and safety problems.34–36
Nicotinic acid is a stable and water soluble vitamin as well as a well-known and inexpensive drug that is known to modulate the activity of different immune cell types such as macrophages, dendritic cells, neutrophils and lymphocytes.37,38 Furthermore, nicotinic acid been reported to play a role in immunomodulation of the gastrointestinal tract but also that it shows an anti-inflammatory capacity in autoimmune disorders through a G protein receptor known as GPR109a.37,39 Despite these interesting properties, application of nicotinic acid for immunomodulatory strategies in the field of regenerative medicine has not yet been explored.
This work therefore presents a new strategy to induce immunomodulatory responses towards implanted biomaterials through incorporation of nicotinic acid in 3D silk scaffolds for bone tissue engineering. Spongy silk scaffolds were fabricated through a freeze-drying process, which was chosen because it allows to easily remove the solvent during the drying step, as there is no need to use surfactants and because it does not require extra washing steps.40,41 Scaffolds were characterized for surface area, porosity, physical stability, water uptake and in vitro biodegradation. Different concentrations of nicotinic acid were loaded into the scaffolds by physical adsorption and the release of incorporated nicotinic acid was monitored over 28 days by UV-Vis spectroscopy. Cytocompatibility was assessed using osteoblast-like MG63 cells and in vitro immune response to the scaffolds was evaluated by monitoring the expression of pro-inflammatory markers by pro-inflammatory M1-like macrophages. To the best of our knowledge, there is no report regarding the production of native silk scaffolds as 3D spongy constructs for a possible application in tissue engineering undertaken without a degumming process. We hypothesize that this approach can be used to construct biomaterials with immunomodulatory properties based on the use of nicotinic acid as an anti-inflammatory drug with potential applications in the field of tissue engineering.
2. Materials and methods
2.1. Preparation of silk scaffolds
Three-dimensional silk scaffolds were prepared using Bombyx mori cocoons. Briefly, 2.5 g cocoon fiber were dissolved in 20 mL 11.5 M LiBr solution for 2 h at 50 °C, dialyzed for 3 days against deionized water using a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
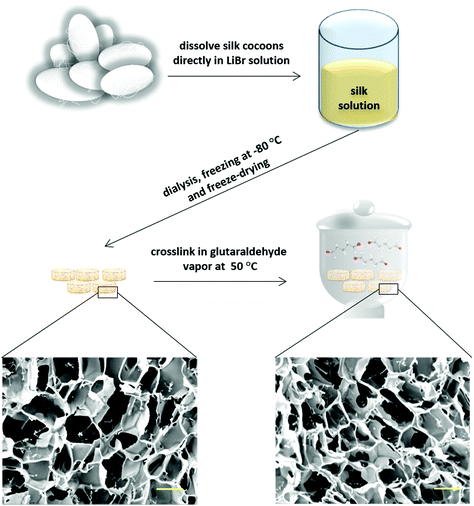
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da cellulose membrane to remove LiBr, removed from the dialysis membrane and stirred for 1 min in a 50 °C water bath to obtain a homogenous solution. After filtering the solution through a filter with 11 μm pore size, it was cooled down in an ice bath to 0 °C, transferred into 48 well plates (500 μL per well), and frozen at −80 °C for 24 h. Finally, the samples were dried in a freeze-dryer (OPERON, FDB-5503) for 3 days and the obtained scaffolds were cut to a size of 2 mm in height using a sharp razor blade. The scaffolds were then cross-linked using glutaraldehyde vapor to increase their stability and degradation resistance. Cross-linking was performed at 50 °C for 18 h using 10 mL glutaraldehyde. After cross-linking, the scaffolds were washed thoroughly in 1% glycine solution to inactivate non-reacted glutaraldehyde. Subsequently, they were immersed in 100% ethanol for 1 min and dried overnight at room temperature.
000 Da cellulose membrane to remove LiBr, removed from the dialysis membrane and stirred for 1 min in a 50 °C water bath to obtain a homogenous solution. After filtering the solution through a filter with 11 μm pore size, it was cooled down in an ice bath to 0 °C, transferred into 48 well plates (500 μL per well), and frozen at −80 °C for 24 h. Finally, the samples were dried in a freeze-dryer (OPERON, FDB-5503) for 3 days and the obtained scaffolds were cut to a size of 2 mm in height using a sharp razor blade. The scaffolds were then cross-linked using glutaraldehyde vapor to increase their stability and degradation resistance. Cross-linking was performed at 50 °C for 18 h using 10 mL glutaraldehyde. After cross-linking, the scaffolds were washed thoroughly in 1% glycine solution to inactivate non-reacted glutaraldehyde. Subsequently, they were immersed in 100% ethanol for 1 min and dried overnight at room temperature.
2.2. Scanning electron microscopy (SEM)
Cross-sections of the 3D foam scaffold were sputter-coated with a 5 nm gold layer before observing the scaffold morphology by scanning electron microscopy (SEM, AIS 2100, Seron Technology) at constant 15 kV accelerating voltage. The average pore size of the scaffolds was measured from 3 SEM images per sample via the software program ImageJ.2.3. Surface area, porosity and density
The surface area of the scaffolds was investigated by the Brunauer, Emmett and Teller (BET) method using a Micromeritics 3Flex Surface Area and Porosity Analyzer. Before the measurement, approximately 100 mg of the scaffolds were degassed at 105 °C for 20 h at a pressure of 1.3 × 10−2 mbar. The adsorption and desorption of the Kr isotherms were collected at 77 K. The relative pressure (P/P0) range was set between 0.02 and 0.62. The sample was measured three times in order to get reproducible results.To calculate the porosity (Φ) of the scaffolds following equation was used:
| Φ = 1 − (ρ/ρskeleton) |
The pore volume (Vpore) of the sample was calculated according to the following equation:
| Vpore = (1/ρ) − (1/ρskeleton). |
2.4. Mechanical properties
The compressive test was carried out on cylindrical-shaped scaffolds with 6 mm diameter and 10 mm height using a compression instrument (Zwick/Roell Z050, Germany). Samples were pre-wetted in PBS overnight prior to the experiment and compression was carried out at room temperature at a crosshead speed of 1 mm min−1 until obtaining 50% of the initial height.2.5. Swelling and in vitro degradation
The swelling of the scaffolds was determined from dry weight (Wd) and wet weight (Ww) after 24 h incubation in deionized water and was calculated according to following equation:| Swelling (%) = [(Ww − Wd)/Wd] × 100. |
To study the in vitro degradation of the scaffolds, after measuring initial weight (Wi) of the samples, they were immersed in PBS (pH = 7.4) and incubated at 37 °C. After specific time intervals, the samples were washed to remove extra salts and their dry weight was then measured.43 The in vitro degradation was calculated according to the following equation:
| Weight loss (%) = [(Wi − Wd)/Wi] × 100. |
2.6. Nicotinic acid loading and release
The scaffolds were loaded with nicotinic acid by adding 100 μL drug solution to the scaffolds using a micropipette. Concentrations of the loading solutions were chosen based on the assumption that a complete release of the drug in 1 mL cell culture medium would result in final concentrations of 1, 5, 10 and 12 mM. After complete absorption of the loading solution, the scaffolds were lyophilized and sample types were termed SNP1, SNP5, SNP10, SNP12 and SC (non-loaded scaffold) (Table 1).| Sample code | Nicotinic acid concentration (mM) | Amount of added nicotinic acid per scaffold (mg) |
|---|---|---|
| SC | 0 | 0 |
| SNP1 | 1 | 0.1231 |
| SNP5 | 5 | 0.615 |
| SNP10 | 10 | 1.231 |
| SNP12 | 12 | 1.846 |
The release study from drug-loaded scaffolds was carried out by soaking the samples in 5 mL PBS at 37 °C in 15 ml Falcon tubes under slow shaking conditions (100 rpm) on a rotary shaker. At different time points, 200 μL of the release medium was withdrawn for OD measurement and replaced with 200 μL fresh PBS. This process was continued for up to 28 days. The amount of nicotinic acid released from the scaffolds was determined using a UV-Vis spectrophotometer (Bio-Tek Plate Reader, Synergy MX, USA) by measuring OD at 262 nm.
2.7. Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR)
The chemical bonding of the scaffolds, before and after drug loading, was characterized by Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy (Varian 640-IR Spectrometer) over the range of 400–4000 cm−1.2.8. Cytotoxicity
To assess potential cytotoxic effects of the scaffolds, the release of lactate dehydrogenase (LDH) from human osteosarcoma cell line (MG63, ATCC® CRL-1427™) was measured using a CytoTox 96 assay (Promega) according to the manufacturer's instructions. In brief, after sterilizing the scaffolds by UV light, they were pre-wetted using 200 μL Minimal Essential Medium Eagle (MEM, Sigma) medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin/neomycin (PSN) and 1% L-glutamine. After incubation for 24 h under standard conditions (37 °C under 5% CO2 and 95% humidity), 88 × 103 osteoblast-like MG63 cells were seeded onto each scaffold. Cell-seeded scaffolds were incubated for 1 and 7 days under standard conditions. Non-loaded scaffolds were used as control. A medium change was performed after 3 days of seeding. Before harvesting the supernatant of the scaffolds at day 1 and 7, 50 μL lysis solution were added into a non-loaded control group containing 500 μL medium and incubated 45 min to prepare a maximum LDH release condition as positive control. After harvesting the supernatants, 50 μL Cytotox96 Reagent were added to 50 μL supernatant of the scaffolds and shaken in the dark for 30 min. After adding 50 μL of stop solution to all samples, absorbance was measured at 490 nm using a Mithras2 LB 943 Multimode Microplate Reader (Berthold Technologies, Germany).To assess the cytocompatibility of soluble nicotinic acid, different concentrations of nicotinic acid in complete MEM medium were prepared and metabolic activity was measured using a resazurin-based PrestoBlue assay according to the manufacturer's instructions. Briefly, 5 × 104 and 2.5 × 104 MG63 cells were seeded in 96-well plates for measurements at day 1 and day 3, respectively. Then, the medium was replaced with nicotinic acid-containing medium for 1 and 3 days before adding 10 μL PrestoBlue Reagent to each well. Absorbance was measured after 15 min at a wavelength of 570 nm.
2.9. THP-1 cell culture
Human monocytic leukemia cell line (THP-1, ECACC 88081201) was used to study the inflammatory response to the scaffolds. After UV sterilization of the scaffolds, samples were pre-wetted in 200 μL RPMI-1640 medium supplemented with 10% FBS, 1% PSN, 1% L-glutamine overnight. THP-1 cells were differentiated into macrophages in complete RPMI-1640 medium supplemented with 100 nM phorbol 12-myristate 13-acetate (PMA, Sigma). After 3 days exposure, the medium was changed and attached cells were incubated in fresh PMA-free medium. After 24 h, the cells were detached using TripLE™ (Gibco Life Technologies) and seeded onto the scaffolds at a concentration of 4 × 105 cells per scaffold in the presence of 1 mL M1 polarizing medium (complete RPMI-1640 medium supplemented with 20 ng mL−1 purified recombinant human interferon γ1b (IFN-γ, MACS, Miltenyi Biotec) and 100 ng mL−1 bacterial lipopolysaccharide (LPS, Sigma-Aldrich). Non-loaded scaffolds in complete RPMI-1640 medium were also used as M0 control.Experiments with the same setup were also performed by seeding the cells onto 2D tissue culture polystyrene in the absence of scaffolds to assess the anti-inflammatory activity of soluble nicotinic acid alone in cell culture medium at final concentrations of 1, 5, 10 and 12 mM (sample codes: NA1, NA5, NA10, and NA12, respectively). Drug-free medium was used as control group (sample code: TCP).
2.10. Cell attachment
Attachment of MG63 and THP-1-derived M1-like macrophages to the scaffolds was evaluated by immunocytochemical staining. The sterilized and pre-wetted samples were seeded with MG63 cells (88 × 103) for 1 and 7 days in MEM medium and M1-like macrophages (4 × 105) for 1 day in RPMI medium containing LPS (100 ng mL−1) and IFN-γ (20 ng mL−1). After each time point, the samples were harvested and washed three times with PBS. The cells were fixed in 4% paraformaldehyde for 30 min, washed with PBS and permeabilized in 0.1% Triton X-100 for 30 min at room temperature. Subsequently, samples were washed 2 times with PBS and then immersed in 1% BSA solution for 30 min to block unspecific binding. Scaffolds were rinsed again with PBS and stained with phalloidin 633 (Alexa Fluor® 633 phalloidin, Molecular Probes®, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution) and DAPI (Sigma, 1
200 dilution) and DAPI (Sigma, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4000 dilution) in 1% BSA on a shaker for 2 h in the dark. After rinsing twice with 1% BSA and twice with PBS, samples were imaged by confocal microscopy (LSM780, Carl Zeiss) using 405 nm and 633 nm laser lines for excitation.
4000 dilution) in 1% BSA on a shaker for 2 h in the dark. After rinsing twice with 1% BSA and twice with PBS, samples were imaged by confocal microscopy (LSM780, Carl Zeiss) using 405 nm and 633 nm laser lines for excitation.
2.11. RNA isolation and RT-PCR
The effect of nicotinic acid on inflammatory response of macrophages was assessed by reverse transcription-polymerase chain reaction (RT-PCR) to measure gene expression levels of TNF-α, CXCL10, CD197 and IL-10. Total RNA from macrophage-seeded scaffolds was isolated after 24 h using RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. The purity of the final RNA product was assessed immediately after isolation by a spectrophotometer at 260 and 280 nm, considering a 260/280 ratio of 1.9–2.1 as pure RNA. RNA samples were reverse transcribed to complementary DNA (cDNA) using iScript cDNA synthesis Kit (BioRad) following the manufacturer's protocol. RT-PCR was performed in a C1000™ Thermal Cycler using the iQ™ SYBR Green Supermix kit in accordance with the manufacturer's instruction. The forward and reverse primer sequences used in this study are summarized in Table 2. The thermal profile of the RT-PCR started with initial denaturation at 95 °C for 3 min, followed by 40 cycles at 95 °C for 10 s (denaturation) and 57 °C for 30 s (annealing). Monitoring the melting curve was performed at 60–95 °C with a temperature increase rate of 0.5 °C per step. Relative expression of the genes was calculated after normalization to ribosomal protein L37a (RPL37a) as housekeeping gene using the 2−ΔΔCt method.| Gene | Abbreviation | PCR primers (5′–3′) |
|---|---|---|
| Tumor necrosis factor alpha | TNF-α | Fw. CTTTGGAGTGATCGGCCCC |
| Rv. GGTTATCTCTCAGCTCCACGC | ||
| Interleukin 10 | IL-10 | Fw. ACATCAAGGCGCATGTGAAC |
| Rv. CAGGGAAGAAATCGATGACAGC | ||
| C–X–C motif chemokine 10 | CXCL10 | Fw. CAGTCTCAGCACCATGAATCAA |
| Rv. CAGTTCTAGAGAGAGGTACTCCTTG | ||
| C–C chemokine receptor type 7 | CD197 | Fw. GTGGTTTTACCGCCCAGAGA |
| Rv. CACTGTGGTGTTGTCTCCGA | ||
| Ribosomal protein L37a | RPL37a | Fw. ATTGAAATCAGCCAGCACGC |
| Rv. AGGAACCACAGTGCCAGATCC |
2.12. DNA quantification and cytokine secretion
To measure DNA content and cytokine secretion, the supernatant of macrophages cultivated on the scaffolds was collected and replaced with 250 μL water 24 h after cell seeding. DNA was isolated from the scaffolds using 3 sequential freeze–thawing cycles (freezing at −20 °C and thawing at room temperature). A Hoechst 33258 assay (Sigma-Aldrich) was performed for DNA quantification. Briefly, 100 μL of the obtained DNA solution were transferred to a 96-well plate and mixed with 100 μL Hoechst solution in TNE buffer (10 mM Tris; 2 M NaCl; 1 mM EDTA; pH 7.4). The fluorescence was measured with 350/460 nm excitation/emission wavelengths after 1 h shaking at room temperature using a Mithras2 LB 943 Multimode Microplate Reader. A DNA standard curve was obtained from a serial dilution of calf thymus DNA (Sigma, D-3664).The concentration of secreted TNF-α in the collected supernatant was determined using a TNF-alpha Human ELISA Kit (Invitrogen) according to the manufacturer's instructions. Optical density was measured with a spectrophotometer at the wavelengths 570 and 450 nm and the data was analyzed after subtracting values of 570 nm from 450 nm wavelength. The concentrations of TNF-α were normalized to the obtained DNA content of each sample.
2.13. Statistical analysis
Data are reported as mean ± standard deviation (SD), and all statistical analyses were performed by two-way ANOVA using Graph Pad Prism software (CA, USA) with Tukey's multiple comparisons test. Differences were considered as statistically significant with a p value <0.05.3. Results and discussion
3.1. Structural characterization of the silk scaffolds
| Porosity (%) | V pore (cm3 g−1) | BET surface area (m2 g−1) | Envelope density (g cm−3) | Skeleton density (g cm−3) |
|---|---|---|---|---|
| 94.1 | 15.60 | 1.24 | 0.060 | 1.018 |
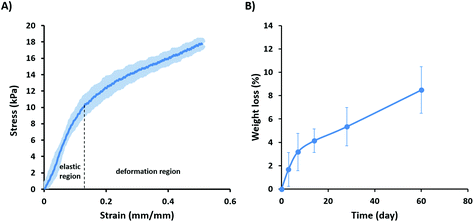
The silk scaffolds were further characterized by measuring their swelling upon immersion in PBS for 24 h, which resulted in swelling of over 500% (557 ± 32). This high swelling is in accordance with some natural polymer sponges and can be attributed to high porosity of the scaffolds as observed in SEM images and porosity measurements.53,64,65 This indicated that silk scaffolds are able to take up a high amount of physiological fluid, which is a key factor when considering the transport of nutrients into the cells.66
For determining the in vitro degradability of the scaffolds, they were immersed in PBS at 37 °C for different time intervals up to 60 days. The scaffolds displayed a slow degradation rate over 60 days with the weight loss increasing over time from 1.7 ± 1.44% after 1 day to 3.2 ± 1.6, 4.1% ± 1, 5.4 ± 1.6, and 8.48 ± 2% after 7, 14, 28 and 60 days, respectively (Fig. 2B). Rapid degradation of the scaffolds after implantation can lead to their weakening or collapse before sufficient tissue growth occurs, and also the release of degradation products can interfere with tissue healing.67,68 Silk fibroin is considered a non-degradable material by the United States Pharmacopeia (USP) and previous results have shown no degradation over 60 days in PBS.13,69 In contrast, our data clearly indicated that scaffolds made of native silk are degradable, however with a slow degradation rate that is likely attributed to the presence of hydrophilic components such as sericin and seroin.70,71
3.2. Drug loading and release
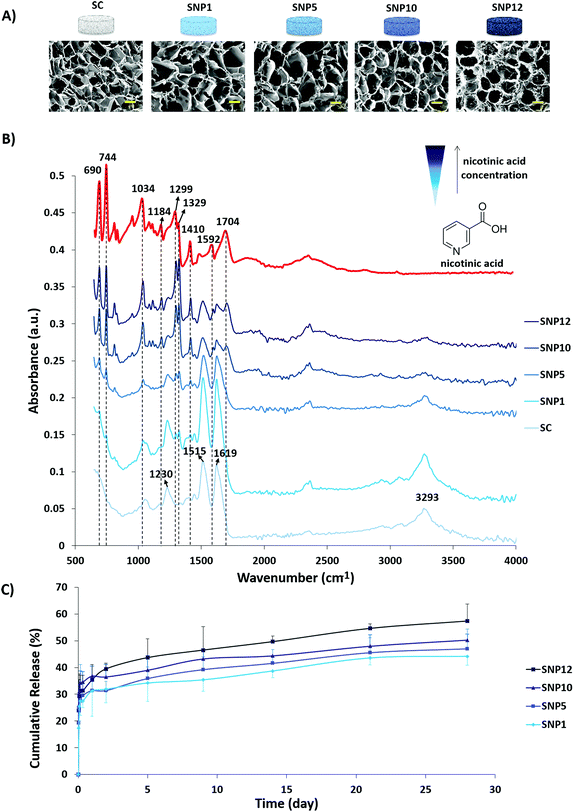
SEM images of the scaffolds after drug loading and corresponding ATR-FTIR spectra as well as cumulative release profiles of nicotinic acid from the scaffolds are shown in Fig. 3. Incorporation of nicotinic acid in the silk scaffolds was performed by adding different concentrations of a nicotinic acid solution drop-wise into the scaffolds with subsequent lyophilization. The advantage of freeze-drying over simple solvent-evaporation is that the former technique avoids aggregation of the drug on the surface due to sublimation, whereas the solvent can carry the drug in latter approach through the capillaries to the surface of the samples due to slow evaporation.72 SEM images of drug-loaded scaffolds (Fig. 3A) demonstrate that no apparent structural change occurred during loading and subsequent lyophilization.Based on ATR-FTIR results (Fig. 3B), an increase in the intensity of nicotinic acid peaks was observed with increasing concentrations of nicotinic acid in the loading solution. The FTIR spectra of the non-loaded scaffold at 1619 (C–H stretching vibration or N–H bending), 1515 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and 1230 cm−1 (C–N stretching or C
O stretching) and 1230 cm−1 (C–N stretching or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending vibration) can be attributed sequentially to amide I, amide II and amide III indicating the presence of silk proteins based on previous reports.73–75 Moreover, the peak at 3293 cm−1 can be related to stretching vibration of the OH group.75 The appearance of sharp peaks around 690, 744, 1299 and 1322 cm−1 can be attributed to C–H deformation vibration band of the pyridine ring of nicotinic acid and ring vibrations. In addition, the peaks at 1034, 1592 and 1704 cm−1 can be assigned to C–O stretching, C
O bending vibration) can be attributed sequentially to amide I, amide II and amide III indicating the presence of silk proteins based on previous reports.73–75 Moreover, the peak at 3293 cm−1 can be related to stretching vibration of the OH group.75 The appearance of sharp peaks around 690, 744, 1299 and 1322 cm−1 can be attributed to C–H deformation vibration band of the pyridine ring of nicotinic acid and ring vibrations. In addition, the peaks at 1034, 1592 and 1704 cm−1 can be assigned to C–O stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations and C
N stretching vibrations and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, respectively.76 As a result, these data indicate that nicotinic acid can be successfully incorporated within the scaffold through lyophilization with no negative structural effect on the scaffolds.
O stretching, respectively.76 As a result, these data indicate that nicotinic acid can be successfully incorporated within the scaffold through lyophilization with no negative structural effect on the scaffolds.
The release behavior of nicotinic acid was investigated after incubating the scaffolds in PBS at 37 °C on a shaker and was quantified using UV-Vis spectroscopy at different time intervals up to 28 days (Fig. 3C). An initial burst release (30–35%) from all drug loaded samples was observed within the first 24 h and was followed by a sustained release reaching 44–57% at day 28. An increase in the rate and amount of released drug was observed with increasing nicotinic acid concentration within the scaffolds as follows: SNP12 > SNP10 > SNP5 > SNP1. The initial burst release of nicotinic acid can be attributed to the free drug close to the surface of the scaffolds and is a common phenomenon in many drug delivery systems.77,78 One possible explanation for the sustained slow release of the drug might be the ability of pyridine and carboxylic acid groups of nicotinic acid to form cyclic hydrogen bonds.79,80 Slow release of biomolecules from silk scaffolds without using any conjugating agent was also reported by others.81,82 Uebersax and colleagues reported slow release of nerve growth factor (NGF) from freeze-dried fibroin matrices within 22 days and attributed this slow rate to possible interactions between fibroin and NGF.83 Furthermore, it has been shown that the degumming process and subsequent decrease in molecular weight of fibroin has a significant effect on its drug delivery profile.84,85 For instance, Fang and colleagues found that with increasing molecular weight of silk hydrogels, the drug release rate from the hydrogels significantly decreases. Furthermore, they observed a stronger diffusion barrier property for the hydrogels with hydrophilic solutes compared to hydrophobic solutes.85 Since the degumming process is omitted from the present research, the slow release of nicotinic acid after the initial burst release is potentially due to the high molecular weight of both fibroin and sericin within the structure. The release model after initial burst release for drug-loaded scaffolds is displayed in Table 4 and based on the obtained relevant coefficients of release kinetics, the best fit for explaining the drug release from the scaffolds follows the Higuchi model, which is mainly considered as a diffusion-controlled mechanism.43,86
| Model | SNP1 | SNP5 | SNP10 | SNP12 |
|---|---|---|---|---|
| Higuchi | 0.964 | 0.988 | 0.981 | 0.990 |
| Zero-order | 0.968 | 0.940 | 0.962 | 0.938 |
| First-order | 0.962 | 0.912 | 0.947 | 0.900 |
| Hixon–Crowel | 0.965 | 0.922 | 0.952 | 0.914 |
| Korsmeyer–Peppas | 0.895 | 0.962 | 0.924 | 0.985 |
3.3. Cell viability and attachment to the silk scaffolds
To investigate cytocompatibility and cell attachment on silk scaffolds, osteoblast-like MG63 cells, one of the most widely studied cell types for osteogenic differentiation, have been employed. MG63 cells were seeded on the silk scaffolds and LDH assay and actin/DAPI staining were performed on day 1 and day 7 (Fig. 4). Furthermore, metabolic activity of the cells in the presence of different concentrations of nicotinic acid in culture medium (0–20 mM) at day 1 and day 3 was evaluated using a PrestoBlue assay.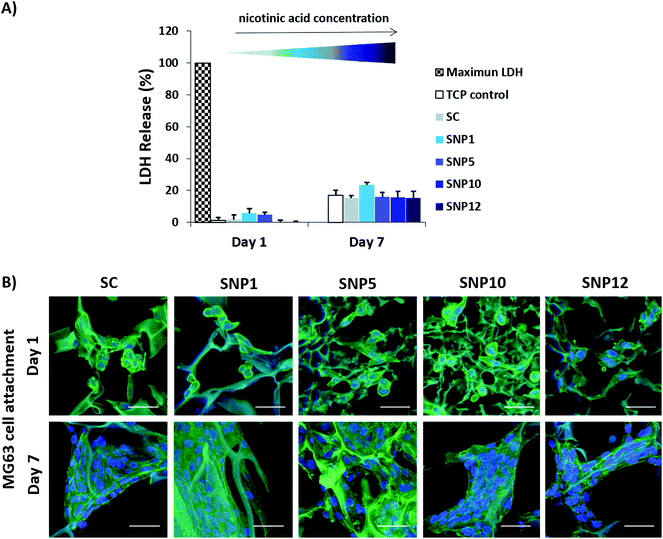 | ||
| Fig. 4 MG63 cell survival on nicotinic acid-loaded silk scaffolds. (A) Cytotoxicity was assessed by measuring LDH release at day 1 and day 7 as an indicator of cell death. (B) Confocal images of MG63 cells seeded on the scaffolds after 7 days of culture stained for F-actin filaments (green)91 and cell nuclei (blue). Scale bars = 50 μm. | ||
All samples showed very low cytotoxicity with LDH values of 0–6% and 15–24% at day 1 and day 7, respectively (Fig. 4A). Notably, no significant difference was detected compared to tissue culture polystyrene (TCP) control groups at both time points. CLSM images showed that cell attachment and spreading was supported on all scaffolds with no apparent difference in cell morphology (Fig. 4B, ESI Fig. S3†). Although most of the cells displayed a round morphology as single cells at day 1, cells were well spread with visible cell-to-cell contact by day 7. Moreover, confocal images also indicated significant cell proliferation from day 1 to day 7.
To further characterize metabolic activity of MG63 cells in soluble nicotinic acid medium (0.5–20 mM), a PrestoBlue assay was performed (ESI Fig. S2†). Low concentrations of nicotinic acid in the cell culture medium showed an increase in metabolic activity compared to drug-free medium. Metabolic activity reached a maximum of 120% at day 1 and 177% at day 3 at 1 mM, which slowly decreased with increasing nicotinic acid concentration. A considerable enhancement in metabolic activity was also detected from day 1 to day 3. Notably, concentrations up to 12 mM nicotinic acid can be considered non-toxic for the cells, showing a metabolic activity >85% at day 1 and >103% at day 3. At higher concentrations (15 mM and higher) metabolic activity drops below 75%, indicating toxic effects of nicotinic acid at elevated concentrations.
Good cell attachment and cytocompatibility are basic requirements when designing tissue engineering scaffolds, as they directly influence cell ingrowth and tissue regeneration. LDH assay and confocal microscopy indicated that all scaffolds show none or negligible toxicity while also supporting cell attachment and growth of MG63 cells. This result is in good agreement with previous reports regarding cytocompatibility of silk proteins.87,88 Moreover, the ability of nicotinic acid in improving cell viability and protecting them from apoptosis has been reported in previous studies.89,90 Overall, these findings suggest that silk scaffolds are able to support cell survival, adhesion and proliferation, and adding different concentrations of nicotinic acid up to 12 mM does not have a negative effect on cytocompatibility.
3.4. Immunomodulation of nicotinic acid-loaded silk scaffolds
Overexpression of pro-inflammatory cytokines and chemokines like TNF-α and CXCL10 after implantation initiate a series of events which lead to secondary immune responses and subsequent tissue damage.92,93 TNF-α for example is known as one of the most important pro-inflammatory cytokines produced by stimulated macrophages and it has been reported that TNF-α and CXCL10 can actively stimulate bone resorption around implanted biomaterials by increasing inflammation and osteoclastogenesis. On the other hand, neutralization of CXCL10 can reduce T cell recruitment and subsequent secondary tissue damages.94–97 Therefore, modulating the secretion of these pro-inflammatory markers likely improves the performance of biomaterials after implantation.To determine the immunomodulatory effect of the silk scaffolds, expression and secretion of inflammatory markers were measured by RT-PCR (Fig. 5) and ELISA (Fig. 6). Gene expression of pro-inflammatory markers TNF-α, CXCL10 and CD197 was assessed after cultivating THP-1-derived M1-like macrophages for 24 h on both non-loaded and nicotinic acid-loaded silk scaffolds. Naïve and M1-like macrophages on non-loaded silk scaffolds served as control groups (SC (Mϕ) and SC (M1), respectively). M1-like macrophages cultivated on silk scaffolds loaded with different concentration of nicotinic acid showed similar cell attachment regardless of nicotinic acid concentration (Fig. 5A). This was confirmed by DNA quantification showing comparable values for all groups without statistically significant differences regarding cell number (ESI Fig. S5†).
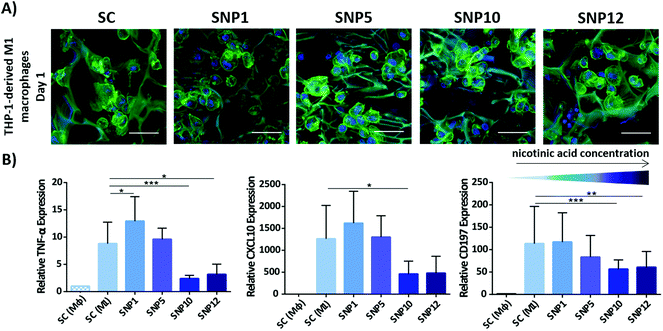 | ||
| Fig. 5 Attachment of M1-like macrophages to silk scaffolds and relative gene expression of inflammatory markers after 24 h. (A) Confocal microscopy of M1-like macrophages seeded on the scaffolds stained for actin filaments (green)91 and cell nuclei (blue). Scale bars = 50 μm. (B) Relative expression of pro-inflammatory markers TNF-α, CXCL10 and CD197. Expression levels ±SD were normalized to Mϕ macrophages seeded on drug-free silk scaffolds (SC (Mϕ)). RPL37a was used as a housekeeping gene. n = 3 (***p < 0.001, **p < 0.01, *p < 0.05). | ||
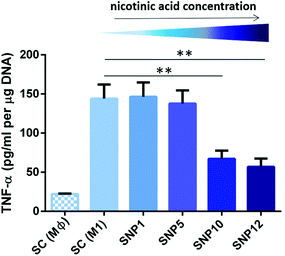 | ||
| Fig. 6 TNF-α secretion from THP-1-derived M1-like macrophages seeded on the scaffolds after 24 h as determined by ELISA and DNA content. (±SD, n = 3) (**p < 0.01). | ||
Gene expression analysis demonstrated that TNF-α expression, one of the major pro-inflammatory cytokines mostly secreted by activated M1 macrophages, is significantly decreased at high nicotinic acid concentrations (samples SNP10 and SNP12) compared to control. Although sample SNP5 showed a similar level of TNF-α gene expression as the non-loaded silk scaffold SC (M1), sample SNP1, with the lowest nicotinic acid concentration, showed a minor, but statistically significant increase in TNF-α level compared to SC (M1). Notably, M1-like macrophages cultivated on TCP in presence of soluble nicotinic acid alone showed only at the highest concentrations (NA12) a significant down-regulation (ESI Fig. S4†). This might be due to potentially higher local concentrations at the surface of the scaffolds when compared to the cells in 2D plates exposed to soluble nicotinic acid in the absence of scaffolds.
The expression level of pro-inflammatory chemokine CXCL10 showed a similar pattern like TNF-α in response to the scaffolds. Only samples with the highest nicotinic acid concentrations (SNP10 and SNP12) effectively downregulated CXCL10, whereas sample SNP5 did not influence the degree of cell polarization compared to the control group. This is in agreement with the obtained data from soluble nicotinic acid in the absence of scaffolds, where medium containing 10 and 12 mM nicotinic acid (NA10 and NA12) displayed a comparable response as samples SNP10 and SNP12 (ESI Fig. S4†). CD197 is also a typical pro-inflammatory marker and was examined to assess the influence of the scaffolds on the expression of M1-like specific macrophage CD markers. Similar to TNF-α and CXCL10, the expression of CD197 was suppressed on samples loaded with higher concentrations of nicotinic acid (SNP10 and SNP12). No significant difference was found in the level of TNF-α, CXCL10, and CD197 between sample SNP10 and SNP12. However, cells in 2D plates exposed to soluble nicotinic acid in the absence of scaffolds showed similar CD197 expression level regardless of nicotinic acid concentration, which contrasts the observed decrease in CD197 expression on loaded scaffolds with increasing nicotinic acid concentration.
The differences in cell response between soluble nicotinic acid in the absence of scaffolds and scaffolds loaded with nicotinic acid points towards an indirect role of the scaffolds in anti-inflammatory activity, as macrophages are known to be influenced by substrate properties.98,99 For example spatial confinement of macrophages, as it occurs on 3D scaffolds when compared to flat 2D surfaces, was recently shown to down-regulate pro-inflammatory responses.99 Also, due to a slow release of the drug from the scaffold into the medium, the local concentration of nicotinic acid on the surface of the scaffolds can be much higher than the soluble nicotinic acid in the medium in the absence of scaffolds, which can in turn increase the anti-inflammatory effect of the drug on the cells. However, as likely both effects contributed to the observed cell response, it is difficult to elucidate the individual contribution of scaffold geometry and local drug concentration.
The release of pro-inflammatory marker TNF-α from M1-like macrophages was also assessed on the protein level (Fig. 6) and paralleled the data from gene expression analysis, with TNF-α being decreased in macrophages on samples SNP10 and SNP12 (67 and 57 pg mL−1, respectively), but not in SNP1 and SNP5, both being comparable to SC (M1) with values of 135–145 pg mL−1. Only the increased gene expression level of TNF-α with sample SNP1 could not be seen on the protein level. Overall, final TNF-α concentration showed the following trend: SC (M1) ≈ SNP1 ≈ SNP5 > SNP10 > SNP12.
The obtained results regarding the anti-inflammatory activity of nicotinic acid is in agreement with a previous report that showed a downregulation of TNF-α expression after exposing ox-LDL-stimulated THP-1-derived macrophages to 0.25–1 mM nicotinic acid for 24 h.100 The concentration difference compared to our observations might be attributed to the nature of the molecules used for macrophage polarization (i.e. LPS and IFN-γ versus ox-LDL) that potentially require a higher concentration of nicotinic acid to downregulate expression of pro-inflammatory markers. The influence of nicotinic acid on the expression of anti-inflammatory marker IL-10 is conflicting, with reports demonstrating a downregulation of IL-10 upon treatment with nicotinic acid.101,102 In the present study, similar expression levels of IL-10 were observed in all experimental groups, demonstrating that nicotinic acid is not able to shift M1-like macrophages to M2-like macrophages in the presence of LPS and IFN-γ (ESI Fig. S6†).
Taken together, these data indicate that nicotinic acid is able to suppress pro-inflammatory markers TNF-α, CXCL10 and CD197 in a concentration dependent manner and scaffold loading with 10 and 12 mM nicotinic acid efficiently suppresses inflammation induced by LPS and IFN-γ. Loading with lower concentrations (i.e. 1 and 5 mM nicotinic acid) is however not sufficient to exert an anti-inflammatory activity. One limitation of this study was however the observed drug release profile with the large initial burst release of nicotinic acid, which would likely only provide a short term anti-inflammatory activity of the scaffold. This should be addressed in future work and well before a potential translation into clinical applications.
4. Conclusion
In the present study, we developed 3D silk scaffolds without prior degumming, successfully loaded them with nicotinic acid to achieve an immunomodulatory biomaterial and investigated their properties and interaction with MG63 cells and human macrophages. We have shown that even without degumming process, silk scaffolds meet a number of design criteria of tissue engineering by offering high porosity, high water absorption capacity and slow degradation rate with excellent cytocompatibility and attachment of MG63 cells. Furthermore, we have obtained evidence that nicotinic acid loaded scaffolds significantly suppressed IFN-γ/LPS-induced expression of pro-inflammatory markers TNF-α, CXCL10 and CD197 at concentrations of 10–12 mM nicotinic acid. While further work is needed to achieve a release profile with long-term efficiency of nicotinic acid in vivo, this study demonstrates the potential of designing immunomodulatory scaffolds that locally release nicotinic acid for minimizing the foreign body reaction for future tissue engineering applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Yvonne Elbs-Glatz and Ursina Tobler for their valuable support in cell culture, RT-PCR and ELISA. This research was partially supported by the Iran National Science Foundation (INSF) Grant No. 96000394.References
- M. M. Alvarez, J. C. Liu, G. Trujillo-de Santiago, B. H. Cha, A. Vishwakarma, A. M. Ghaemmaghami and A. Khademhosseini, Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications, J. Controlled Release, 2016, 240, 349–363 CrossRef CAS PubMed.
- L. Chung, D. R. Maestas Jr., F. Housseau and J. H. Elisseeff, Key players in the immune response to biomaterial scaffolds for regenerative medicine, Adv. Drug Delivery Rev., 2017, 114, 184–192 CrossRef CAS PubMed.
- Y. Onuki, U. Bhardwaj, F. Papadimitrakopoulos and D. J. Burgess, A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response, J. Diabetes Sci. Technol., 2008, 2, 1003–1015 CrossRef.
- T. Desai and L. D. Shea, Advances in islet encapsulation technologies, Nat. Rev. Drug Discovery, 2017, 16, 338–350 CrossRef CAS PubMed.
- Z. Chen, T. Klein, R. Z. Murray, R. Crawford, J. Chang, C. Wu and Y. Xiao, Osteoimmunomodulation for the development of advanced bone biomaterials, Mater. Today, 2016, 19, 304–321 CrossRef CAS.
- A. Vishwakarma, N. S. Bhise, M. B. Evangelista, J. Rouwkema, M. R. Dokmeci, A. M. Ghaemmaghami, N. E. Vrana and A. Khademhosseini, Engineering immunomodulatory biomaterials to tune the inflammatory response, Trends Biotechnol., 2016, 34, 470–482 CrossRef CAS PubMed.
- J. S. Lewis, K. Roy and B. G. Keselowsky, Materials that harness and modulate the immune system, MRS Bull., 2014, 39, 25–34 CrossRef.
- R. M. Boehler, J. G. Graham and L. D. Shea, Tissue engineering tools for modulation of the immune response, BioTechniques, 2011, 51, 239–240 CrossRef CAS.
- S. Kapoor and S. C. Kundu, Silk protein-based hydrogels: Promising advanced materials for biomedical applications, Acta Biomater., 2016, 31, 17–32 CrossRef CAS PubMed.
- H. Kweon, S. G. Kim and J. Y. Choi, Inhibition of foreign body giant cell formation by 4- hexylresorcinol through suppression of diacylglycerol kinase delta gene expression, Biomaterials, 2014, 35, 8576–8584 CrossRef CAS PubMed.
- S. Sapru, S. Das, M. Mandal, A. K. Ghosh and S. C. Kundu, Prospects of nonmulberry silk protein sericin-based nanofibrous matrices for wound healing - In vitro and in vivo investigations, Acta Biomater., 2018, 78, 137–150 CrossRef CAS PubMed.
- M. Bhattacharjee, E. Schultz-Thater, E. Trella, S. Miot, S. Das, M. Loparic, A. R. Ray, I. Martin, G. C. Spagnoli and S. Ghosh, The role of 3D structure and protein conformation on the innate and adaptive immune responses to silk-based biomaterials, Biomaterials, 2013, 34, 8161–8171 CrossRef CAS.
- G. H. Altman, F. Diaz, C. Jakuba, T. Calabro, R. L. Horan, J. Chen, H. Lu, J. Richmond and D. L. Kaplan, Silk-based biomaterials, Biomaterials, 2003, 24, 401–416 CrossRef CAS.
- F. Javed, M. Al-Askar, K. Almas, G. E. Romanos and K. Al-Hezaimi, Tissue reactions to various suture materials used in oral surgical interventions, ISRN Dent., 2012, 2012, 762095 Search PubMed.
- L. S. Wray, X. Hu, J. Gallego, I. Georgakoudi, F. G. Omenetto, D. Schmidt and D. L. Kaplan, Effect of processing on silk-based biomaterials: reproducibility and biocompatibility, J. Biomed. Mater. Res., Part B, 2011, 99, 89–101 CrossRef.
- K. Nultsch, L. K. Bast, M. Näf, S. E. Yakhlifi, N. Bruns and O. Germershaus, Effects of silk degumming process on physicochemical, tensile, and optical properties of regenerated silk fibroin, Macromol. Mater. Eng., 2018, 303, 1800408 CrossRef.
- L. Wang, Z. Luo, Q. Zhang, Y. Guan, J. Cai, R. You and X. Li, Effect of degumming methods on the degradation behavior of silk fibroin biomaterials, Fibers Polym., 2019, 20, 45–50 CrossRef CAS.
- B. J. Allardyce, R. Rajkhowa, R. J. Dilley, M. D. Atlas, J. Kaur and X. Wang, The impact of degumming conditions on the properties of silk films for biomedical applications, Text. Res. J., 2016, 86, 275–287 CrossRef CAS.
- F. Ak, Z. Oztoprak, I. Karakutuk and O. Okay, Macroporous silk fibroin cryogels, Biomacromolecules, 2013, 14, 719–727 CrossRef CAS.
- E. Mariani, G. Lisignoli, R. M. Borzì and L. Pulsatelli, Biomaterials: foreign bodies or tuners for the immune response?, Int. J. Mol. Sci., 2019, 20, 636 CrossRef CAS.
- I. Cantón, R. Mckean, M. Charnley, K. A. Blackwood, C. Fiorica, A. J. Ryan and S. MacNeil, Development of an Ibuprofen-releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration, Biotechnol. Bioeng., 2010, 105, 396–408 CrossRef.
- M. Shi, Z. Chen, S. Farnaghi, T. Friis, X. Mao, Y. Xiao and C. Wu, Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis, Acta Biomater., 2016, 30, 334–344 CrossRef CAS PubMed.
- M. Shi, L. Xia, Z. Chen, F. Lv, H. Zhu, F. Wei, S. Han, J. Chang, Y. Xiao and C. Wu, Europium-doped mesoporous silica nanosphere as an immune-modulating osteogenesis/angiogenesis agent, Biomaterials, 2017, 144, 176–187 CrossRef CAS PubMed.
- Z. Chen, J. Yuen, R. Crawford, J. Chang, C. Wu and Y. Xiao, The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate, Biomaterials, 2015, 61, 128–138 Search PubMed.
- J. M. Morais, F. Papadimitrakopoulos and D. J. Burgess, Biomaterials/tissue interactions: possible solutions to overcome foreign body response, AAPS J., 2010, 12, 188–196 CrossRef CAS.
- R. M. Boehler, R. Kuo, S. Shin, A. G. Goodman, M. A. Pilecki, R. M. Gower, J. N. Leonard and L. D. Shea, Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-Inflammatory phenotype, Biotechnol. Bioeng., 2014, 111, 1210–1221 CrossRef CAS.
- J. M. H. Liu, J. Zhang, X. Zhang, K. A. Hlavaty, C. F. Ricci, J. N. Leonard, L. D. Shea and R. M. Gower, Biomaterials transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets, Biomaterials, 2016, 80, 11–19 CrossRef CAS.
- W. K. Ward, J. C. Hansen, R. G. Massoud, J. M. Engle, M. M. Takeno and K. D. Hauch, Controlled release of dexamethasone from subcutaneously-implanted biosensors in pigs: localized anti-inflammatory benefit without systemic effects, J. Biomed. Mater. Res., Part A, 2010, 94, 280–287 CrossRef.
- Q. Wang, H. Li, Y. Xiao, S. Li, B. Li, X. Zhao, L. Ye, B. Guo, X. Chen, Y. Ding and C. Bao, Locally controlled delivery of TNFα antibody from a novel glucose-sensitive scaffold enhances alveolar bone healing in diabetic conditions, J. Controlled Release, 2015, 28, 232–242 CrossRef.
- D. Dzhonova, R. Olariu, J. Leckenby, A. Dhayani, P. K. Vemula, J. C. Prost, Y. Banz, A. Taddeo and R. Rieben, Local release of tacrolimus from hydrogel-based drug delivery system is controlled by inflammatory enzymes in vivo and can be monitored non-invasively using in vivo imaging, PLoS One, 2018, 13, e0203409 CrossRef.
- W. Wang, L. Sun, P. Zhang, J. Song and W. Liu, An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits, Acta Biomater., 2014, 10, 4983–4995 CrossRef CAS.
- Y. Liu, L. Wang, T. Kikuiri, K. Akiyama, C. Chen, X. Xu, R. Yang, W. Chen, S. Wang and S. Shi, Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α, Nat. Med., 2011, 17, 1594–1601 CrossRef CAS.
- D. P. Vasconcelos, M. Costa, I. F. Amaral, M. A. Barbosa, A. P. Águas and J. N. Barbosa, Development of an immunomodulatory biomaterial: Using resolvin D1 to modulate inflammation, Biomaterials, 2015, 53, 566–573 CrossRef CAS.
- K. Rehman, M. S. Hamid Akash, B. Akhtar, M. Tariq, A. Mahmood and M. Ibrahim, Delivery of therapeutic proteins: challenges and strategies, Curr. Drug Targets, 2016, 17, 1172–1188 CrossRef CAS.
- M. C. Robert, M. Frenette, C. Zhou, Y. Yan, J. Chodosh, F. A. Jakobiec, A. M. Stagner, D. Vavvas, C. H. Dohlman and E. I. Paschalis, A drug delivery system for administration of anti–TNF-α antibody, Transl. Vis. Sci. Technol., 2016, 5, 11 CrossRef.
- K. Haynes, T. Beukelman, J. R. Curtis, C. Newcomb, L. J. Herrinton, D. J. Graham, D. H. Solomon, M. R. Griffin, L. Chen, L. Liu, K. G. Saag and J. D. Lewis, Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases, Arthritis Rheum., 2013, 65, 48–58 CrossRef CAS.
- N. Singh, A. Gurav, S. Sivaprakasam, E. Brady, R. Padia, H. Shi, M. Thangaraju, P. D. Prasad, S. Manicassamy, D. H. Munn, J. R. Lee, S. Offermanns and V. Ganapathy, Activation of the receptor (Gpr109a) for niacin and the commensal metabolite butyrate suppresses colonic inflammation and carcinogenesis, Immunity, 2014, 40, 128–139 CrossRef CAS.
- W. J. Lee and K. Hase, Gut microbiota-generated metabolites in animal health and disease, Nat. Chem. Biol., 2014, 10, 416–424 CrossRef CAS.
- H. A. Salem and W. Wadie, Effect of niacin on inflammation and angiogenesis in a murine model of ulcerative colitis, Sci. Rep., 2009, 7, 7139 CrossRef.
- N. Annabi, J. W. Nichol, X. Zhong, C. Ji, S. Koshy, A. Khademhosseini and F. Dehghani, Controlling the porosity and microarchitecture of hydrogels for tissue engineering, Tissue Eng., Part B, 2010, 16, 371–383 CrossRef CAS.
- H. M. Yin, J. Qian, J. Zhang, Z. F. Lin, J. S. Li, J. Z. Xu and Z. M. Li, Engineering porous poly(lactic acid) scaffolds with high mechanical performance via a solid state extrusion/porogen leaching approach, Polymers, 2016, 8, 213–226 CrossRef.
- C. Brewer, V. J. Chuang, C. A. Masiello, H. Gonnermann, X. Gao, B. Dugan, L. E. Driver, P. Panzacchi, K. Zygourakis and C. A. Davies, New approaches to measuring biochar density and porosity, Biomass Bioenergy, 2014, 66, 176–185 CrossRef CAS.
- S. Dash, P. N. Murthy, L. Nath and P. Chowdhury, Kinetic modeling on drug release from controlled drug delivery systems, Acta Pol. Pharm., 2010, 67, 217–223 CAS.
- A. Di Luca, B. Ostrowska, I. Lorenzo-Moldero, A. Lepedda, W. Swieszkowski, C. Van Blitterswijk and L. Moroni, Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds, Sci. Rep., 2016, 6, 22898 CrossRef CAS.
- G. P. Huang, S. Shanmugasundaram, P. Masih, D. Pandya, S. Amara, G. Collins and T. L. Arinzeh, An investigation of common crosslinking agents on the stability of electrospun collagen scaffolds, J. Biomed. Mater. Res., Part A, 2015, 103, 762–771 CrossRef.
- J. Ratanavaraporn, R. Rangkupan, H. Jeeratawatchai, S. Kanokpanont and S. Damrongsakkul, Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats, Int. J. Biol. Macromol., 2010, 47, 431–438 CrossRef CAS.
- A. G. Destaye, C. K. Lin and C. K. Lee, Glutaraldehyde vapor cross-linked nanofibrous PVA mat with in situ formed silver nanoparticles, ACS Appl. Mater. Interfaces, 2013, 5, 4745–4752 CrossRef CAS.
- C. M. Murphy and F. J. O'Brien, Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds, Cell Adhes. Migr., 2010, 4, 377–381 CrossRef.
- I. Bružauskaitė, D. Bironaitė, E. Bagdonas and E. Bernotienė, Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects, Cytotechnology, 2016, 68, 355–369 CrossRef.
- G. Hannink and J. J. Arts, Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration?, Injury, 2011, 42, S22–S25 CrossRef.
- B. A. Harley, H. D. Kim, M. H. Zaman, I. V. Yannas, D. A. Lauffenburger and L. J. Gibson, Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions, Biophys. J., 2008, 95, 4013–4024 CrossRef CAS.
- C. M. Murphy, M. G. Haugh and F. J. O'Brien, The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering, Biomaterials, 2010, 31, 461–466 CrossRef CAS.
- S. Zeng, L. Liu, Y. Shi, J. Qiu, W. Fang, M. Rong, Z. Guo and W. Gao, Characterization of silk fibroin/chitosan 3D porous scaffold and in vitro cytology, PLoS One, 2015, 10, e0128658 CrossRef.
- B. B. Mandal and S. C. Kundu, Cell proliferation and migration in silk fibroin 3D scaffolds, Biomaterials, 2009, 30, 2956–2965 CrossRef CAS.
- G. Weibrich, R. Trettin, S. H. Gnoth, H. Götz, H. Duschner and W. Wagner, Determining the size of the specific surface of bone substitutes with gas adsorption, Mund Kiefer Gesichtschir., 2000, 4, 148–152 CrossRef CAS.
- S. Tassani, C. Ohman, F. Baruffaldi, M. Baleani and M. Viceconti, Volume to density relation in adult human bone tissue, J. Biomech., 2011, 44, 103–108 CrossRef PubMed.
- A. Nisal, R. Sayyad, P. Dhavale, B. Khude, R. Deshpande, V. Mapare, S. Shukla and P. Venugopalan, Silk fibroin micro-particle scaffolds with superior compression modulus and slow bioresorption for effective bone regeneration, Sci. Rep., 2018, 8, 7235 CrossRef.
- D. Panda, S. Konar, S. K. Bajpai and A. Arockiarajan, Synthesis and viscoelastic characterization of microstructurally aligned Silk fibroin sponges, J. Mech. Behav. Biomed. Mater., 2017, 71, 362–371 CrossRef CAS.
- W. H. Elliott, W. Bonani, D. Maniglio, A. Motta, W. Tan and C. Migliaresi, Silk hydrogels of tunable structure and viscoelastic properties using different chronological orders of genipin and physical cross-linking, ACS Appl. Mater. Interfaces, 2015, 7, 12099–12108 CrossRef CAS.
- N. Huebsch, P. R. Arany, A. S. Mao, D. Shvartsman, O. A. Ali, S. A. Bencherif, J. Rivera-Feliciano and D. J. Mooney, Harnessing traction-mediated manipulation of the cell-matrix interface to control stem cell fate, Nat. Mater., 2010, 9, 518–526 CrossRef CAS.
- G. Chen, C. Dong, L. Yang and Y. Lv, 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering, ACS Appl. Mater. Interfaces, 2015, 7, 15790–15802 CrossRef CAS.
- S. L. Levengood and M. Zhang, Chitosan-based scaffolds for bone tissue engineering, J. Mater. Chem. B, 2014, 2, 3161–3184 RSC.
- G. Tozzi, A. De Mori, A. Oliveira and M. Roldo, Composite hydrogels for bone regeneration, Materials, 2016, 9, 267 CrossRef.
- J. Li, Q. Wang, Y. Gu, Y. Zhu, L. Chen and Y. Chen, Production of composite scaffold containing silk fibroin, chitosan, and gelatin for 3D cell culture and bone tissue regeneration, Med. Sci. Monit., 2017, 23, 5311–5320 CrossRef.
- J. Liu, D. Meisner, E. Kwong, X. Y. Wu and M. R. Johnston, A novel trans-lymphatic drug delivery system: implantable gelatin sponge impregnated with PLGA-paclitaxel microspheres, Biomaterials, 2007, 28, 3236–3244 CrossRef CAS.
- K. Maji, S. Dasgupta, K. Pramanik and A. Bissoyi, Preparation and evaluation of gelatin-chitosan-nanobioglass 3D porous scaffold for bone tissue engineering, Int. J. Biomater., 2016, 2016, 9825659 Search PubMed.
- D. Noviana, D. Paramitha, M. F. Ulum and H. Hermawan, The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats, J. Orthop. Translat., 2015, 9, 9–15 Search PubMed.
- M. Yazdimamaghania, M. Razavia, D. Vashaeeb and L. Tayebi, Development and degradation behavior of magnesium scaffolds coated with polycaprolactone for bone tissue engineering, Mater. Lett., 2014, 132, 106–110 CrossRef.
- R. L. Horan, K. Antle, A. L. Collette, Y. Wang, J. Huang, J. E. Moreau, V. Volloch, D. L. Kaplan and G. H. Altman, In vitro degradation of silk fibroin, Biomaterials, 2005, 26, 3385–3393 CrossRef CAS.
- J. W. Kim, Y. Y. Jo, H. Y. Kweon, D. W. Kim and S. G. Kim, The effects of proteins released from silk mat layers on macrophages, Maxillofac. Plast. Reconstr. Surg., 2018, 40, 10 CrossRef.
- H. Teramoto, A. Kakazu, K. Yamauchi and T. Asakura, Role of hydroxyl side chains in bombyx mori silk sericin in stabilizing its solid structure, Macromolecules, 2007, 40, 1562–1569 CrossRef CAS.
- S. W. Song, H. J. Bae, S. Kim, D. Y. Oh, O. Kim, Y. Jeong and S. Kwon, Uniform drug loading into prefabricated microparticles by freeze-drying, Part. Part. Syst. Charact., 2017, 34, 1600427 CrossRef.
- S. W. Ha, A. E. Tonelli and S. M. Hudson, Structural studies of Bombyx mori silk fibroin during regeneration from solutions and wet fiber spinning, Biomacromolecules, 2005, 6, 1722–1731 CrossRef CAS.
- D. W. Li, X. Lei, F. L. He, J. He, Y. L. Liu, Y. J. Ye, X. Deng, E. Duan and D. C. Yin, Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells, Int. J. Biol. Macromol., 2017, 105, 584–597 CrossRef CAS.
- J. Nourmohammadi, F. Roshanfar, M. Farokhi and M. Haghbin Nazarpak, Silk fibroin/kappa-carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications, Mater. Sci. Eng., C, 2017, 76, 951–958 CrossRef CAS.
- G. Socrates, Infrared and raman characteristic group frequencies: tables and charts, John Wieley and Sons, 2001, pp. 168–171 Search PubMed.
- T. N. Vo, F. K. Kasper and A. G. Mikos, Strategies for controlled delivery of growth factors and cells for bone regeneration, Adv. Drug Delivery Rev., 2012, 64, 1292–1309 CrossRef CAS.
- K. J. Rambhia and P. X. Ma, Controlled drug release for tissue engineering, J. Controlled Release, 2015, 219, 119–128 CrossRef CAS.
- S. H. Dale, M. R. J. Elsegood, M. Hemmings and A. L. Wilkinson, The co-crystallisation of pyridine with benzenepolycarboxylic acids: The interplay of strong and weak hydrogen bonding motifs, CrystEngComm, 2004, 6, 207–214 RSC.
- S. J. Ford, G. J. McIntyre, M. R. Johnson and I. R. Evans, Structure and dynamics studies of the short strong hydrogen bond in the 3,5-dinitrobenzoic acid–nicotinic acid molecular complex, CrystEngComm, 2013, 15, 7576–7582 RSC.
- S. Hofmann, C. T. Foo, F. Rossetti, M. Textor, G. Vunjak-Novakovic, D. L. Kaplan, H. P. Merkle and L. Meinel, Silk fibroin as an organic polymer for controlled drug delivery, J. Controlled Release, 2006, 111, 219–227 CrossRef CAS.
- B. B. Mandal, S. Kapoor and S. C. Kundu, Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release, Biomaterials, 2009, 30, 2826–2836 CrossRef CAS.
- L. Uebersax, M. Mattotti, M. Papaloïzos, H. P. Merkle, B. Gander and L. Meinel, Silk fibroin matrices for the controlled release of nerve growth factor (NGF), Biomaterials, 2007, 28, 4449–4460 CrossRef CAS.
- E. M. Pritchard, X. Hu, V. Finley, C. K. Kuo and D. L. Kaplan, Effect of silk protein processing on drug delivery from silk films, Macromol. Biosci., 2013, 13, 311–320 CrossRef CAS.
- J. Y. Fang, J. P. Chen, Y. L. Leu and H. Y. Wang, Characterization and evaluation of silk protein hydrogels for drug delivery, Chem. Pharm. Bull., 2006, 54, 156–162 CrossRef CAS.
- Y. Fu and W. J. Kao, Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems, Expert Opin. Drug Delivery, 2010, 7, 429–444 CrossRef CAS.
- A. Varkey, E. Venugopal, P. Sugumaran, G. Janarthanan, M. M. Pillai, S. Rajendran and A. Bhattacharyya, Impact of silk fibroin-based scaffold structures on human osteoblast MG63 cell attachment and proliferation, Int. J. Nanomed., 2015, 10, 43–51 CAS.
- V. Mitrana, M. G. Albub, E. Vasilec, A. Cimpeana and M. Costache, Dose-related effects of sericin on preadipocyte behavior within collagen/sericin hybrid scaffolds, Prog. Nat. Sci.: Mater. Int., 2015, 25, 122–130 CrossRef.
- K. Weidele, A. Kunzmann, M. Schmitz, S. Beneke and A. Bürkle, Ex vivo supplementation with nicotinic acid enhances cellular poly(ADP-ribosyl)ation and improves cell viability in human peripheral blood mononuclear cells, Biochem. Pharmacol., 2010, 80, 1103–1112 CrossRef CAS.
- X. Dou, C. Shen, Z. Wang, S. Li, X. Zhang and Z. Song, Protection of nicotinic acid against oxidative stress-induced cell death in hepatocytes contributes to its beneficial effect on alcohol-induced liver injury in mice, J. Nutr. Biochem., 2013, 24, 1520–1528 CrossRef CAS.
- R. C. de Guzman, M. R. Merrill, J. R. Richter, R. I. Hamzi, O. K. Greengauz-Roberts and M. E. Van Dyke, Mechanical and biological properties of keratose biomaterials, Biomaterials, 2011, 32, 8205–8217 CrossRef CAS PubMed.
- R. Gonzalez, J. Glaser, M. T. Liu, T. E. Lane and H. S. Keirstead, Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury, Exp. Neurol., 2003, 184, 456–463 CrossRef CAS.
- J. R. Bradley, TNF-mediated inflammatory disease, J. Pathol., 2008, 214, 149–160 CrossRef CAS.
- E. M. Schwarz, R. J. Looney and R. J. O'Keefe, Anti-TNF-α therapy as a clinical intervention for periprosthetic osteolysis, Arthritis Res., 2000, 2, 165–168 CrossRef CAS.
- J. Lam, S. Takeshita, J. E. Barker, O. Kanagawa, F. P. Ross and S. L. Teitelbaum, TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand, J. Clin. Invest., 2000, 106, 1481–1488 CrossRef CAS.
- E. Jämsen, V. P. Kouri, J. Olkkonen, A. Cör, S. B. Goodman, Y. T. Konttinen and J. Pajarinen, Characterization of macrophage polarizing cytokines in the aseptic loosening of total hip replacements, J. Orthop. Res., 2014, 32, 1241–1246 CrossRef.
- A. D. Schoenenberger, A. Schipanski, V. Malheiro, M. Kucki, J. G. Snedeker, P. Wick and K. Maniura-Weber, Macrophage polarization by titanium dioxide (TiO2) particles: size matters, ACS Biomater. Sci. Eng., 2016, 2, 908–919 CrossRef CAS.
- X. H. Qin, B. Senturk, J. Valentin, V. Malheiro, G. Fortunato, Q. Ren, M. Rottmar and K. Maniura-Weber, Cell membrane-inspired silicone interfaces that mitigate pro-inflammatory macrophage activation and bacterial adhesion, Langmuir, 2018, 8b02292 Search PubMed.
- N. Jain and V. Vogel, Spatial confinement downsizes the inflammatory response of macrophages, Nat. Mater., 2018, 17, 1134–1144 CrossRef CAS.
- Y. Si, Y. Zhang, J. Zhao, S. Guo, L. Zhai, S. Yao, H. Sang, N. Yang, G. Song, J. Gu and S. Qin, Niacin inhibits vascular inflammation via downregulating nuclear transcription factor-κB signaling pathway, Mediators Inflammation, 2014, 2014, 263786 Search PubMed.
- S. Montserrat-de la Paz, M. C. Naranjo, S. Lopez, R. Abia, F. J. G. Muriana and B. Bermudez, Niacin and its metabolites as master regulators of macrophage activation, J. Nutr. Biochem., 2017, 39, 40–47 CrossRef CAS.
- D. Wanders, E. C. Graff, B. D. White and R. L. Judd, Niacin increases adiponectin and decreases adipose tissue inflammation in high fat diet-fed mice, PLoS One, 2013, 8, e71285 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9bm00814d |
| This journal is © The Royal Society of Chemistry 2020 |