Stimulated Raman scattering by intracavity mixing of nanosecond laser excitation and fluorescence in acoustically levitated droplets†
Andreas
Bierstedt
,
Carsten
Warschat
,
Yi
You
,
Knut
Rurack
 and
Jens
Riedel
and
Jens
Riedel
 *
*
Bundesanstalt für Materialforschung und -prüfung (BAM), Richard-Willstätter-Straße 11, 12489 Berlin, Germany. E-mail: Jens.Riedel@bam.de; Fax: +4930810471003; Tel: +493081041003
First published on 30th September 2020
Abstract
Raman spectroscopy is becoming a commonly used, powerful tool for structural elucidation and species identification of small liquid samples, e.g. in droplet-based digital microfluidic devices. Due to the low scattering cross sections and the temporal restrictions dictated by the droplet flow, however, it depends on amplification strategies which often come at a cost. In the case of surface-enhanced Raman scattering (SERS), this can be an enhanced susceptibility towards memory effects and cross talk, whereas resonant and/or stimulated Raman techniques require higher instrumental sophistication, such as tunable lasers or the high electromagnetic field strengths which are typically provided by femtosecond lasers. Here, an alternative instrumental approach is discussed, in which stimulated Raman scattering (SRS) is achieved using the single fixed wavelength output of an inexpensive diode-pumped solid-state (DPSS) nanosecond laser. The required field strengths are realized by an effective light trapping in a resonator mode inside the interrogated droplets, while the resonant light required for the stimulation is provided by the fluorescence signal of an admixed laser dye. To elucidate the underlying optical processes, proof-of-concept experiments are conducted on acoustically levitated droplets, mimicking a highly reproducible and stable digital fluidic system. By using isotope-labeled compounds, the assignment of the emitted radiation as Raman scattering is firmly corroborated. A direct comparison reveals an amplification of the usually weak spontaneous Stokes emission by up to five orders of magnitude. Further investigation of the optical power dependence reveals the resulting gain to depend on the intensity of both, the input laser fluence and the concentration of the admixed fluorophore, leaving SRS as the only feasible amplification mechanism. While in this study stable large droplets have been studied, the underlying principles also hold true for smaller droplets, in which case significantly lower laser pulse energy is required. Since DPSS lasers are readily available with high repetition rates, the presented detection strategy bears a huge potential for fast online identification and characterization routines in digital microfluidic devices.
Introduction
High throughput chemical and biological screening approaches often rely on the compartmentalization of liquids into separate droplets inside a non-miscible medium. Typically, these droplets are in continuous motion and for subsequent analysis they individually propagate into and out of the interrogation volume by either a carrier medium flow or gravitational force. Consequently, the dwell time in the sampling cross section determines the upper temporal limit for the characterization. The most commonly used realization of this so-called concept of “digital microfluidics” are micrometer-sized droplets inside microfluidic chips and flow cytometry.1–3 For both methods, contactless optical characterization methods, such as absorption4 or fluorescence spectroscopy5 are most commonly used. In addition, Raman spectroscopy can also serve as a powerful characterization tool to elucidate the chemical nature of droplets. However, the Raman scattering response is typically low within the given temporal window. Raman spectroscopy usually requires long integration times, a darkened laboratory environment, and an effective notch filter to suppress the residual light of the excitation laser. Moreover, any competitive optical emission, e.g., unwanted fluorescence, often masks the weak Raman bands, limiting its application range in microfluidic devices.Several amplification strategies to enhance the intensity of Raman scattering have been reported, among which the local amplification of electromagnetic field strengths in the close vicinity of electron-rich nanostructures, i.e., surface-enhanced Raman spectroscopy (SERS),6 is the most prominent due to its high sensitivity and multiplex detection capability.7–12 As a consequence, various SERS-based microfluidic systems have been reported recently, in which these nanostructures are directly inserted into microfluidic channels. Following this approach, however, memory effects and cross talk between individual droplets has been observed due to nanoparticle aggregation on the channel surfaces.13–15 To overcome this obstacle, stimulated Raman scattering has been implemented in digital microfluidics.3 This process requires a rather complex two-color double-resonant femtosecond excitation instrumentation, which stands in strong contrast to the inexpensive and portable character of microfluidic chips. A promising route towards lower instrumental sophistication involves fluorescence enhancement of stimulated Raman in liquid-core optical fibers, in which a fluorescence photon is used to seed the stimulated transition of the radiative stokes relaxation in the Raman process.16 Here, the weak Raman scattering can build up from a strong fluorescence band. In the past, this effect has been applied mainly in hollow-core fibers,17,18 which provide a much longer path for the interaction between the light and the sample as compared to the case of single droplets.
To study the interaction between single droplets and the excitation light, stationary droplets are beneficial. Throughout the last years, acoustically levitated droplets have received attention as potential microfluidic reactors and as compartmentalized containers for analytical sampling.19–23 As such, they pose as ideal, stationary test-beds for droplet interrogation schemes. While along the literature, the contactless analysis of levitated droplets has been achieved by different techniques, including mass spectrometry,24–26 ion mobility spectrometry,27 X-ray,28 and UV/vis spectroscopy,29 the most prominent analytical technique is Raman spectroscopy.21,30,31
Spherical liquid droplets exhibit a whispering gallery mode (WGM),32,33 which can act as an optical resonator. While resonant excitation of WGM has become a standard tool in micrometer-sized solid34 and liquid35 spheres, only a few experiments have been reported on optical pumping of fluorophore-doped liquid droplets on the millimeter scale.36–38 Different authors independently observed the occurrence of a narrow, red-shifted bright emission inside the droplet above a well-defined threshold, which has been ascribed to different optical phenomena. Since power dependence studies beyond the threshold revealed gain behavior, in the referenced publications, the emission is commonly referred to as lasing. Still, a comprehensive explanation for the experienced red-shift and the spectral pattern of the emission is yet missing. This work introduces a Raman based interrogation strategy of droplets on the example of millimeter sized levitated stationary droplets. The use of two amplification methodologies, exploitation of the whispering gallery mode, and stimulation of the emission by using fluorescence as a seed photon, an amplification of five orders of magnitude can be achieved.
Experimental section
Chemicals
Two sets of 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM) (98%, Sigma Aldrich, Steinheim, Germany) containing solutions, spanning a concentration range from 1 × 10−3 M to 1 × 10−9 M, were used to investigate the nonlinear Raman scattering processes induced by intracavity mixing of nanosecond laser excitation and fluorescence in acoustically levitated droplets. Stock solutions of the dye were prepared gravimetrically with a dye mole fraction of 1 × 10−3 M in methanol (HPLC grade, ≥99.85%, Th. Geyer, Renningen, Germany) and ethanol (HPLC grade, ≥99.99%, Fisher Scientific, Loughborough, UK), respectively. Additional sample solutions with concentrations of 1 × 10−4 M, 5 × 10−5 M, 1 × 10−5 M, 5 × 10−6 M, 1 × 10−6 M, 5 × 10−7 M, 1 × 10−7 M, 5 × 10−8 M, 1 × 10−8 M, 5 × 10−9 M, and 1 × 10−9 M were prepared by serial dilution of the stock solutions. As reference samples, the neat organic solvents were used in each case. All chemicals were used without further purification.Acoustic levitation
All experiments were conducted using a commercially available acoustic levitator (Tec 5, Oberursel, Germany). The device is set up in a single axis assembly, comprising a piezo-driven transducer operating at a frequency of 58 kHz, a sonotrode ending in a radiating plate, and an opposing concave reflector. Constructive interference conditions are achieved by placing the reflector 5/2 wavelengths (≈21 mm) from the radiating plate. At these conditions, locally confined areas of elevated pressure (antinodes), intersected by areas at the unaltered pressure of the surroundings (nodes) subsist inside the resonator.39 Inside the nodes, the acoustic force compensates the gravity and, thus, matter of appropriate volume and density can be suspended contactless in air. For each measurement, single droplets with a total volume of 5 μL were manually injected with an Eppendorf pipette inside the central node. Note, that slightly decreasing the resonance distance between the sonotrode and the reflector increased the spatial confinement of the suspended volume, while at the same time a deformation into oblate-shaped droplets was observed. This behavior did not show any significant effect on the spectral appearance or the intensity of the emitted radiation.Optical measurements
All spectra were recorded using the experimental setup shown in Fig. 1. The λ = 1064 nm output of a Nd:YAG DPSS laser (Titan AC 30 MM, SPEKTRUM Laser Entwicklungs- und Vertriebs GmbH, Berlin, Germany) was fed through a beta barium borate (BBO) crystal under the phase matching angle, yielding frequency doubled coherent light of λ = 532 nm with a maximum energy of 12 mJ per pulse and a pulse width of 7 ns at FWHM, at a repetition rate of 15 Hz. The laser beam passed two laser line mirrors (NB1-K13, Thorlabs, Dachau, Germany) to achieve an optimal spatial overlap between the laser beam and the droplet. The laser spot size was determined to be ≈4 mm in diameter, illuminating the entire droplet volume. Residual light of λ = 1064 nm and the λ = 808 nm from the DPSS pumping diode bank were separated by a polarizing harmonic separator. Optical emission was collected perpendicular to the excitation light axis via an optical fiber and sent to the entrance slit of a Czerny–Turner spectrograph (SR-303i-B, 600 grooves per mm grating, Andor Technology Ltd., Belfast, UK), equipped with a CCD camera (iDus, −60 °C, Andor Technology Ltd., Belfast, UK). The obtained spectra are the mean of 50 independent replicate measurements, with 100 ms integration time each. The entrance slit width was set to 150 μm. If necessary, absorptive neutral density filters (ND 1.0 – ND 4.0, Thorlabs, Dachau, Germany) were implemented into the setup to avoid saturation of the detector. For comparison, Raman spectra of larger sample volumes were recorded with the same acquisition optics in a quartz cuvette (Hellma Analytics, 10 mm optical path length) placed into the excitation beam at the same position as the droplet.Results
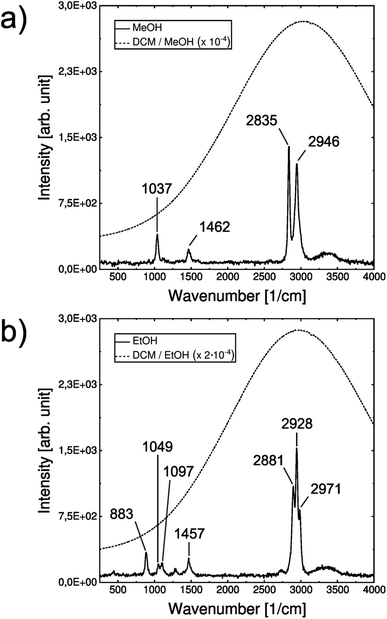
Before the actual experiments on droplets, a characterization of the individual involved transitions was performed. For this purpose, both spontaneous Raman spectra of MeOH and EtOH and the spectral pattern of the fluorescence emission of the laser dye DCM in the respective solvents were recorded in quartz cuvettes. A direct comparison of the two phenomena depicted in Fig. 2 reveals an effective spectral overlap between the most intense Raman bands in the C–H stretching region of the individual vibrational spectra and the respective fluorescence signal. The Raman and fluorescence spectra are depicted as functions of Raman and Stokes shift rather than absolute wavelength. Please note, that this overlap is intentional by choosing the appropriate laser dye. For observations of Raman bands at a different shift, other dyes in that spectral window would be used (see e.g. the Raman spectra of the deuterated isotopologue MeOH d-4 using Pyrromethane 597 instead discussed below and presented in Fig. S1†).For the intracavity experiments, the cuvette was replaced by acoustically levitated droplets of the two alcohols with a fixed concentration of cDCM = 10−5 M. Upon radiation with the pulsed green laser light, a clear superposition of a narrow spectral feature between 2800 and 3000 cm−1 sitting on top of a spectrally broad background can be observed (see Fig. 3).
To emphasize the characteristic emission behavior stemming from the spherical shape of the sample, Fig. 3 also contains the equivalent spectra obtained from sample in cuvettes. The latter are between factors of 15 (EtOH) and 40 (MeOH) weaker in peak intensity and lack the characteristic narrow spectral features. The intensity difference can directly be understood by the droplet acting as a WGM resonator which efficiently traps photons and resonantly enhances the emission. A plausible explanation for the appearance of the narrow bands can be given by referring to Fig. 1b. Inside the optical WGM cavity of the sphere, one photon of an incident narrowband pump beam λP excites the fluorophore, inducing broadband fluorescence λFl, whose energy difference matches the vibrational energy of a particular band resulting in a selective, stimulated inelastic scattering of photons λSRS‡ off from vibrationally excited molecules in solution that interfere coherently. This classification of the narrow feature as stimulated Raman bands is strongly corroborated by the clear similarity of the spectral position and pattern of the bands with the C–H stretch Raman signals shown in Fig. 2. Still, an unambiguous assignment of the enhancement process to stimulated Raman scattering (SRS) needs further substantiation.
Before considering the amplification process, the vibrational transition in Raman scattering has to be confirmed to be responsible for the observed peaks. For that purpose, the same experiment was conducted with a combination of the fully deuterated MeOH-d4 and the fluorophore Pyrromethene 597. As isotopologues, MeOH and MeOH-d4 differ in their vibrational spectra while having the same electronic structures. The dye was rationally selected since it exhibits a Stokes shift resonating with the known ∼2000 cm−1 Raman shift of the C–D stretching vibrations of the deuterated solvent. The corresponding vibrational spectra, including the spontaneous Raman scattering of MeOH-d4, the fluorescence spectrum of pyrromethene 597 in MeOH, and the SRS spectrum of pyrromethene 597 in MeOH-d4 are shown in the ESI in Fig. S1.† The result clearly reveals the absence of the characteristic band structure of Fig. 3 at ca. 3000 cm−1. Instead, a new group of bands with a ∼1000 cm−1 blue-shift become prominent at 2076, 2136, and 2245 cm−1. These values coincide with the Raman bands which have been shifted by the kinetic isotope effect upon H/D exchange. This is strong evidence for C-H/D stretch vibrations to be the amplified optical transition, leaving Raman scattering as the only reasonable explanation for the observed mode and a mere uncertainty regarding the mechanism of the amplification process.
A major difference between spontaneous and stimulated processes is their individual power dependence. Thus, to fortify the presence of an SRS mechanism, a series of spectra with a fixed DCM concentration of cDCM = 1 × 10−5 M for different pump laser pulse energies was recorded. The results are summarized in Fig. 4a and c for MeOH and EtOH, respectively. The reason for the unfavorable signal-to-noise ratio in Fig. 4c is an artifact caused by the ND filters, necessary due to the overall brighter emission. The neutral density of the used filters is considered in the values depicted in the figure. Again, a direct comparison with Fig. 2 allows for an assignment of the two spectral features as the C–H Raman contribution and the fluorescence of the admixed DCM laser dye. To extract the power dependence of the optical response, Fig. 4b and d show the signal intensity (ISRS) as the peak height of the Raman contribution after subtraction of the underlying fluorescence band intensity as a function of the pump laser pulse energy EP. Details on the quantification and the background subtraction can be found in the ESI.† Briefly, the background corrected values could be obtained from single experiments in a way which allows for a direct quantitative comparison between the individual values of ISRS.
The equatorial emission characteristics along the entire 2π circumference of the spherical droplet leads to only a small fraction of the emission being detected by the solid acceptance angle of the collection fiber. This hampers the determination of absolute efficiency values, however, the slope ∂ISRS/∂EP is directly proportional to the efficiency of the light conversion process. Towards higher laser fluences, the intensity of the Raman signal does at first linearly increase until at a pulse energy of ∼5 mJ per pulse a roughly eight-fold increase in the slope is observed. This behavior is characteristic for the transition from a spontaneous radiative decay with a given efficiency ∂ISRS/∂EP to a stimulated process with an enhanced efficiency. This gain-type change in the optical response curves clearly serves as an indicator for a coherent nonlinear amplification process as the origin of the Raman amplification.
Opposing spontaneous Raman scattering, where the intensity of the emitted wavelength only depends on the number of photons in the pump mode nP, in stimulated Raman processes it also scales with the irradiated Stokes photons nS, being proportional to nP(nS + 1).40 Consequently, in the next set of experiments, the dependence of the Raman gain signal on the Stokes intensity (IS), which is equivalent to the intensity of the fluorescence in the spectral window of the Raman band, was tested. As ISRS, IS is determined as the peak height of the signal at the observed wavelength (for details, see ESI Fig. S2†). For this purpose, the spectrally underlying Raman intensity stemming from the broadband fluorescence (ref. Fig. S2†) of alcoholic droplets with DCM admixtures of different concentrations were determined as the intensity of the Stokes contribution (IS) and used as the abscissa in the Raman gain curves depicted in Fig. 5. The ordinate values represent ISRS. As depicted in Fig. S2,† for a most direct comparison, each individual data pair consisting of a value of IS and ISRS for a given DCM concentration was extracted from the same spectrum.
The individual curves taken under a constant pulse energy of the pump laser at λ = 532 nm of E = 12 mJ per pulse show a pronounced nonlinear response between the integrated SRS signal and the number of photons of the Stokes wavelength nS. Direct comparison between Fig. 5a and b indicate the lasing threshold, i.e., the IS at which the response slope changes, to be different for the two solvents. When compared to the dependence on the pump photon intensity (Ip), the change in the slope ∂ISRS/∂IS is even more pronounced than ∂ISRS/∂EP. Again, a quantitative description is hampered by the fact that no absolute values for the wave intensities can be obtained. However, in this experiment, the fact that both ISRS and IS were collected under identical solid angle conditions, allows for an approximate value of ∼25% for the efficiency from the slope. A comparison between the detected signal intensities in Fig. 5b and 2b shows that in the case of EtOH, when changing from spontaneous to stimulated Raman scattering, the intensity of the Raman band is enhanced by up to five orders of magnitude.
When exploiting spectral resonances, one always enters a tradeoff between sensitivity and selectivity. The latter is especially pronounced in the case of resonant intracavity experiments where the mode structure results in only a well-defined, selected few wavelengths to be active inside the resonator. This phenomenon can be seen when probing the fluorescence response of two different droplets of two different radii and ellipticities, respectively. The ellipticity leads to a continuous spectrum of different radii along different slanting angles inside the droplet. Because resonance occurs if the circumference around the droplet 2πr coincides with an integer multiple of the wavelength. Fig. 6 shows the results for a 5 μL droplet of 5.5 μM DCM ethanol-solution (red curve) and the same droplet after 3 minutes of shrinkage due to evaporation of the ethanol (blue curve). The shadowgraphic images of the droplets in the inset of Fig. 6a show the blue droplet to have a smaller maximum radius and ellipticity leading to a lower number of modes along its internal circumference. The fringe pattern superimposed onto the fluorescence response reveals the coexistence of at least 20 modes, while the spacing (Δλ) and width (δλ) of the spectral bands taken from the Fourier transform of the spectra recalculated to the wavelength domain (Fig. 6b) allow for a lower limit estimation of the finesse of the resonator to  . This is only a lower limit, due to the convolution with the instrument response function of the low-resolution spectrometer, the actual finesse is likely to be much higher. Because the fringes are caused by subsequent wavelengths of constructive and destructive interference, this resonator could only enhance spectral features coinciding with constructive wavelengths while other Raman features would be suppressed. The red curve in Fig. 6 shows that when enhancing the maximum radius and the ellipticity it allows for more modes stably coexisting inside the same resonator until the fringe pattern completely disappears.
. This is only a lower limit, due to the convolution with the instrument response function of the low-resolution spectrometer, the actual finesse is likely to be much higher. Because the fringes are caused by subsequent wavelengths of constructive and destructive interference, this resonator could only enhance spectral features coinciding with constructive wavelengths while other Raman features would be suppressed. The red curve in Fig. 6 shows that when enhancing the maximum radius and the ellipticity it allows for more modes stably coexisting inside the same resonator until the fringe pattern completely disappears.
For an analytical application as show above, a compromise must be found between an effective light trapping and little spectral demand regarding wavelength selectivity. The trapping ensures an enhanced overlap between the interacting wavefunctions (overlap integral) while the latter can be achieved by using multimode resonators.
Discussion
Here, we described how a Raman scattering signal can be enhanced by several orders of magnitude with a single, non-resonant nanosecond excitation laser pulse by exploiting the perfectly spherical shape of droplets and the addition of rationally selected laser dyes, which exhibit a fluorescence shift energetically corresponding to the Stokes shift of the Raman signal of interest. In this way, two waves, the pump laser and the fluorescence, coexist inside a circular resonator along the equatorial periphery of the droplet. The resonator confines both waves into a long interaction volume, providing a temporally large overlap of the two electromagnetic fields. Thus, when the Stokes-shifted emission of the fluorescent dye is in resonance with an inelastic Raman mode of the solvent, an effective coupling can occur. The result is a pronounced SRS process inside the cavity of this whispering gallery resonator, visible as a strong emission along the circumference of the droplet.The results reported here show that while maintaining the enhancement factors typical for SRS, the experimental instrumentation can be kept relatively simple. A single nanosecond pulsed laser and the use of a Stokes-matched laser dye suffices to generate intra-cavity amplification of stimulated Raman scattering inside droplets. Even with their lower finesse caused by more pronounced imperfections, such as ellipticity and surface ripples caused by the ultrasonic carrier wave, the whispering gallery mode resonator provided a large interaction volume for the pump and Stokes wave. The latter may be important because of the relatively short coherence length of the fluorescence. Since the propagation inside the whispering gallery mode resonator can be interpreted as a persisting chain of total internal reflection, Fresnel's law assures that only fluorescence of the right polarization accumulates inside the cavity. The result is an enhancement of the Stokes signal by up to five orders of magnitude. Therefore, in contrast to spontaneous Raman scattering experiments, no notch filter was necessary, the sample could be exposed to ambient light and the presence of fluorescence and scattered light from the excitation pump laser did not compete with the intense SRS signal. This brings SRS into the grasp of analytical applications in compact, robust, and inexpensive integrated microfluidic systems which would greatly benefit from a means for real-time structure elucidation by vibrational spectroscopy.
Conclusion
When illuminating a levitated droplet containing alcoholic dye solution with a single fixed wavelength DPSS nanosecond laser, a bright spectrally narrow signal was observed, which could be unambiguously assigned as stimulated Raman scattering. This effect, which is caused by the nonlinear mixing between the residual light of the pump laser and the resonant fraction of the broadband fluorescence emission inside the whispering gallery mode along the droplet's internal equatorial circumference, was studied regarding the power dependence of the individual input waves. The observed SRS signal was found to depend on both the pump and the Stokes input wave in a highly nonlinear fashion, resulting in an amplification of the usually weak spontaneous Stokes emission by up to five orders of magnitude. While in real applications, this amplification can be used to detect analytes in lower concentration or within a shorter interrogation time, here for direct comparison all spectra were recorded under the same conditions. This enhancement bears the potential for analytical applications of fluorescence-seeded SRS in microfluidic devices targeting either low concentrations or at a short interrogation time. Compared to femtosecond lasers, nanosecond pulsed DPSS lasers are inexpensive, robust, and potentially portable. As such, they reflect all the benefits which microfluidic chips offer in sample management to the instrumentation used for interrogation. On top of that, their availability with high repetition rates of several tens of kilohertz nicely matches the requirements for high throughput characterization of droplet streams.Conflicts of interest
The authors declare no competing financial interest.References
- K. Choi, A. H. C. Ng, R. Fobel and A. R. Wheeler, Digital Microfluidics, in Annual Review of Analytical Chemistry, ed. R. G. Cooks and E. S. Yeung, Annual Reviews, Palo Alto, 2012, vol. 5, pp. 413–440 Search PubMed.
- A. F. Chrimes, K. Khoshmanesh, P. R. Stoddart, A. Mitchell and K. Kalantar-zadeh, Microfluidics and Raman microscopy: current applications and future challenges, Chem. Soc. Rev., 2013, 42(13), 5880–5906 RSC.
- C. Zhang, K.-C. Huang, B. Rajwa, J. Li, S. Yang, H. Lin, C.-s. Liao, G. Eakins, S. Kuang, V. Patsekin, J. P. Robinson and J.-X. Cheng, Stimulated Raman scattering flow cytometry for label-free single-particle analysis, Optica, 2017, 4(1), 103–109 CrossRef CAS.
- V. Srinivasan, V. K. Pamula and R. B. Fair, An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids, Lab Chip, 2004, 4(4), 310–315 RSC.
- B. Kuswandi, N. Nuriman, J. Huskens and W. Verboom, Optical sensing systems for microfluidic devices: a review, Anal. Chim. Acta, 2007, 601(2), 141–155 CrossRef CAS.
- M. Kerker, D. S. Wang and H. Chew, Surface Enhanced Raman-Scattering (Sers) By Molecules Adsorbed At Spherical-Particles, Appl. Opt., 1980, 19(24), 4159–4174 CrossRef CAS.
- B. Tang, J. Wang, J. A. Hutchison, L. Ma, N. Zhang, H. Guo, Z. Hu, M. Li and Y. Zhao, Ultrasensitive, Multiplex Raman Frequency Shift Immunoassay of Liver Cancer Biomarkers in Physiological Media, ACS Nano, 2016, 10(1), 871–879 CrossRef CAS.
- G. C. Phan-Quang, E. H. Z. Wee, F. Yang, H. K. Lee, I. Y. Phang, X. Feng, R. A. Alvarez-Puebla and X. Y. Ling, Online Flowing Colloidosomes for Sequential Multi-analyte High-Throughput SERS Analysis, Angew. Chem., Int. Ed., 2017, 56(20), 5565–5569 CrossRef CAS.
- T. Gong, Z.-Y. Hong, C.-H. Chen, C.-Y. Tsai, L.-D. Liao and K. V. Kong, Optical Interference-Free Surface-Enhanced Raman Scattering CO-Nanotags for Logical Multiplex Detection of Vascular Disease Related Biomarkers, ACS Nano, 2017, 11(3), 3365–3375 CrossRef CAS.
- Z. Cheng, N. Choi, R. Wang, S. Lee, K. C. Moon, S.-Y. Yoon, L. Chen and J. Choo, Simultaneous Detection of Dual Prostate Specific Antigens Using Surface-Enhanced Raman Scattering-Based Immunoassay for Accurate Diagnosis of Prostate Cancer, ACS Nano, 2017, 11(5), 4926–4933 CrossRef CAS.
- C. Zong, M. Xu, L.-J. Xu, T. Wei, X. Ma, X.-S. Zheng, R. Hu and B. Ren, Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges, Chem. Rev., 2018, 118(10), 4946–4980 CrossRef CAS.
- Y. Zeng, J.-Q. Ren, A.-G. Shen and J.-M. Hu, Splicing Nanoparticles-Based “Click” SERS Could Aid Multiplex Liquid Biopsy and Accurate Cellular Imaging, J. Am. Chem. Soc., 2018, 140(34), 10649–10652 CrossRef CAS.
- S. Lin, W. Zhu, Y. Jin and K. B. Crozier, Surface-Enhanced Raman Scattering with Ag Nanoparticles Optically Trapped by a Photonic Crystal Cavity, Nano Lett., 2013, 13(2), 559–563 CrossRef CAS.
- R. Gao, J. Ko, K. Cha, J. H. Jeon, G.-e. Rhie, J. Choi, A. J. deMello and J. Choo, Fast and sensitive detection of an anthrax biomarker using SERS-based solenoid microfluidic sensor, Biosens. Bioelectron., 2015, 72, 230–236 CrossRef CAS.
- R. Gao, Z. Cheng, X. Wang, L. Yu, Z. Guo, G. Zhao and J. Choo, Simultaneous immunoassays of dual prostate cancer markers using a SERS-based microdroplet channel, Biosens. Bioelectron., 2018, 119, 126–133 CrossRef CAS.
- A. S. Kwok and R. K. Chang, Fluorescence Seeding Of Weaker-Gain Raman Modes In Microdroplets - Enhancement Of Stimulated Raman-Scattering, Opt. Lett., 1992, 17(18), 1262–1264 CrossRef CAS.
- Z. W. Men, G. N. Qu, W. H. Fang, X. P. Sun, A. Y. Cao, Z. W. Li, C. L. Sun, S. Q. Gao and G. H. Lu, Continuous wave stimulated Raman scattering of benzene by fluorescence enhancement in hollow fused silica fiber, J. Raman Spectrosc., 2011, 42(7), 1489–1491 CrossRef CAS.
- R. Yang, H. L. Ma and H. P. Jin, Influencing factors of external fluorescence seeding enhancing stimulated Raman scattering in liquid-core optical fiber, J. Raman Spectrosc., 2013, 44(12), 1689–1692 CrossRef CAS.
- Z. Chen, D. Y. Zang, L. Zhao, M. F. Qu, X. Li, X. G. Li, L. X. Li and X. G. Geng, Liquid Marble Coalescence and Triggered Microreaction Driven by Acoustic Levitation, Langmuir, 2017, 33(25), 6232–6239 CrossRef CAS.
- N. J. Mason, E. A. Drage, S. M. Webb, A. Dawes, R. McPheat and G. Hayes, The spectroscopy and chemical dynamics of microparticles explored using an ultrasonic trap, Faraday Discuss., 2008, 137, 367–376 RSC.
- F. Priego-Capote and L. de Castro, Ultrasound-assisted levitation: lab-on-a-drop, TrAC, Trends Anal. Chem., 2006, 25(9), 856–867 CrossRef CAS.
- S. Santesson and S. Nilsson, Airborne chemistry: acoustic levitation in chemical analysis, Anal. Bioanal. Chem., 2004, 378(7), 1704–1709 CrossRef CAS.
- A. Scheeline and R. L. Behrens, Potential of levitated drops to serve as microreactors for biophysical measurements, Biophys. Chem., 2012, 165, 1–12 CrossRef.
- E. A. Crawford, C. Esen and D. A. Volmer, Real Time Monitoring of Containerless Microreactions in Acoustically Levitated Droplets via Ambient Ionization Mass Spectrometry, Anal. Chem., 2016, 88(17), 8396–8403 CrossRef CAS.
- C. Warschat, A. Stindt, U. Panne and J. Riedel, Mass Spectrometry of Levitated Droplets by Thermally Unconfined Infrared-Laser Desorption, Anal. Chem., 2015, 87(16), 8323–8327 CrossRef CAS.
- M. S. Westphall, K. Jorabchi and L. M. Smith, Mass spectrometry of acoustically levitated droplets, Anal. Chem., 2008, 80(15), 5847–5853 CrossRef CAS.
- A. Michalik-Onichimowska, T. Beitz, U. Panne, H.-G. Löhmannsröben and J. Riedel, Laser ionization ion mobility spectrometric interrogation of acoustically levitated droplets, Anal. Bioanal. Chem., 2019, 8053–8061 CrossRef CAS.
- J. Leiterer, W. Leitenberger, F. Emmerling, A. F. Thunemann and U. Panne, The use of an acoustic levitator to follow crystallization in small droplets by energy-dispersive X-ray diffraction, J. Appl. Crystallogr., 2006, 39, 771–773 CrossRef CAS.
- J. Schenk, L. Trobs, F. Emmerling, J. Kneipp, U. Panne and M. Albrecht, Simultaneous UV/Vis spectroscopy and surface enhanced Raman scattering of nanoparticle formation and aggregation in levitated droplets, Anal. Methods, 2012, 4(5), 1252–1258 RSC.
- R. Tuckermann, L. Puskar, M. Zavabeti, R. Sekine and D. McNaughton, Chemical analysis of acoustically levitated drops by Raman spectroscopy, Anal. Bioanal. Chem., 2009, 394(5), 1433–1441 CrossRef CAS.
- B. R. Wood, P. Heraud, S. Stojkovic, D. Morrison, J. Beardall and D. McNaughton, A portable Raman acoustic levitation spectroscopic system for the identification and environmental monitoring of algal cells, Anal. Chem., 2005, 77(15), 4955–4961 CrossRef CAS.
- E. Kim, M. D. Baaske and F. Vollmer, Towards next-generation label-free biosensors: recent advances in whispering gallery mode sensors, Lab Chip, 2017, 17(7), 1190–1205 RSC.
- L. N. He, S. K. Ozdemir and L. Yang, Whispering gallery microcavity lasers, Laser Photonics Rev., 2013, 7(1), 60–82 CrossRef CAS.
- L. Collot, V. Lefevreseguin, M. Brune, J. M. Raimond and S. Haroche, Very High-Q Whispering-Gallery Mode Resonances Observed On Fused-Silica Microspheres, Europhys. Lett., 1993, 23(5), 327–334 CrossRef CAS.
- R. Symes, R. M. Sayer and J. P. Reid, Cavity enhanced droplet spectroscopy: principles, perspectives and prospects, Phys. Chem. Chem. Phys., 2004, 6(3), 474–487 RSC.
- S. K. Y. Tang, Z. Li, A. R. Abate, J. J. Agresti, D. A. Weitz, D. Psaltis and G. M. Whitesides, A multi-color fast-switching microfluidic droplet dye laser, Lab Chip, 2009, 9(19), 2767–2771 RSC.
- S. X. Qian, J. B. Snow, H. M. Tzeng and R. K. Chang, Lasing Droplets - Highlighting The Liquid-Air Interface By Laser-Emission, Science, 1986, 231(4737), 486–488 CrossRef CAS.
- H. Azzouz, L. Alkhafadiji, S. Balslev, J. Johansson, N. A. Mortensen, S. Nilsson and A. Kristensen, Levitated droplet dye laser, Opt. Express, 2006, 14(10), 4374–4379 CrossRef CAS.
- A. Bierstedt, U. Panne and J. Riedel, Confinement and enhancement of an airborne atmospheric laser-induced plasma using an ultrasonic acoustic resonator, J. Anal. At. Spectrom., 2018, 33(1), 135–140 RSC.
- R. W. Hellwarth, Theory of Stimulated Raman Scattering, Phys. Rev., 1963, 130(5), 1850–1852 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ay01504k |
| ‡ Please note for consistency the nomenclature SRS already here refers to stimulated Raman scattering even before the process is unambiguously identified as SRS later in the paper. |
| This journal is © The Royal Society of Chemistry 2020 |