 Open Access Article
Open Access ArticleAmbient (desorption/ionization) mass spectrometry methods for pesticide testing in food: a review
Miriam
Beneito-Cambra
a,
Bienvenida
Gilbert-López
 ab,
David
Moreno-González
c,
Marcos
Bouza
a,
Joachim
Franzke
ab,
David
Moreno-González
c,
Marcos
Bouza
a,
Joachim
Franzke
 c,
Juan F.
García-Reyes
c,
Juan F.
García-Reyes
 ab and
Antonio
Molina-Díaz
ab and
Antonio
Molina-Díaz
 *ab
*ab
aAnalytical Chemistry Research Group (FQM-323), Department of Physical and Analytical Chemistry, University of Jaen, 23071 Jaén, Spain. E-mail: amolina@ujaen.es; Tel: +34 953 212147
bCenter for Advanced Studies in Olives Grove and Olive Oils (CEAOAO), Science and Technology Park GEOLIT, E-23620 Mengíbar, Spain
cISAS—Leibniz Institut für Analytische Wissenschaften, Bunsen-Kirchhoff-Str. 11, 44139 Dortmund, Germany
First published on 2nd September 2020
Abstract
Ambient mass spectrometry refers to the family of techniques that allows ions to be generated from condensed phase samples under ambient conditions and then, collected and analysed by mass spectrometry. One of their key advantages relies on their ability to allow the analysis of samples with minimal to no sample workup. This feature maps well to the requirements of food safety testing, in particular, those related to the fast determination of pesticide residues in foods. This review discusses the application of different ambient ionization methods for the qualitative and (semi)quantitative determination of pesticides in foods, with the focus on different specific methods used and their ionization mechanisms. More popular techniques used are those commercially available including desorption electrospray ionization (DESI-MS), direct analysis on real time (DART-MS), paper spray (PS-MS) and low-temperature plasma (LTP-MS). Several applications described with ambient MS have reported limits of quantitation approaching those of reference methods, typically based on LC-MS and generic sample extraction procedures. Some of them have been combined with portable mass spectrometers thus allowing “in situ” analysis. In addition, these techniques have the ability to map surfaces (ambient MS imaging) to unravel the distribution of agrochemicals on crops.
1. Introduction
Pesticides are plant protection products intended for preventing, destroying, repelling or mitigating any pest (harmful organisms, such as insects, fungi, or weeds, among others) or disease. They may also influence the life processes of plants (e.g. growth regulators, nitrogen stabilizers) or preserve crops during production, storage and transport.1,2 Annual pesticide sales in the period 2011–2016 were close to 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of active ingredients, only in the European Union (EU).3 As a consequence of this extended use, their residues may be found in foods of both vegetable and animal origin, and also as pollutants in the environment.4,5 In order to asses food safety and to reduce any risk to human and animal health arising from pesticide exposure, pesticide residues have been restricted in developed countries. Public organizations such as the EU, the United States Environmental Protection Agency (USEPA) or Codex Alimentarius have established maximum residue levels (MRLs) permitted in food, taking into consideration the acceptable daily intake of pesticides (amount of pesticide ingested daily during the whole life without leading to noticeable adverse effects).6
000 tons of active ingredients, only in the European Union (EU).3 As a consequence of this extended use, their residues may be found in foods of both vegetable and animal origin, and also as pollutants in the environment.4,5 In order to asses food safety and to reduce any risk to human and animal health arising from pesticide exposure, pesticide residues have been restricted in developed countries. Public organizations such as the EU, the United States Environmental Protection Agency (USEPA) or Codex Alimentarius have established maximum residue levels (MRLs) permitted in food, taking into consideration the acceptable daily intake of pesticides (amount of pesticide ingested daily during the whole life without leading to noticeable adverse effects).6
This framework fosters the development of analytical methods enabling the detection of pesticides at concentration levels below the MRLs set.7 Multiresidue methods, the preferred option for food analysis, rely on hyphenated techniques such as gas chromatography/mass spectrometry (GC-MS) or high performance liquid chromatography/mass spectrometry (HPLC-MS).6 Nowadays, the feasibility of real-time pesticide testing, performed “in situ”, with little or no sample preparation and avoiding the chromatographic separation step, remains a challenge which attracts the attention of food safety researchers. This greener approach, which fulfils many Green Analytical Chemistry principles, is feasible using ambient MS techniques as captured in Fig. 1.8
 | ||
| Fig. 1 Typical workflow of a routine pesticide testing method using chromatographic techniques and the role ambient MS may play to speed up these procedures, allowing even on-site sample analysis when portable MS instrumentation is used. Adapted from ref. 8 with permission from Elsevier. | ||
Ambient MS9 is a rapidly growing field started with the development of desorption electrospray ionization (DESI)10 and direct analysis in real time (DART).11 Since its inception, over eighty different ambient MS approaches have been proposed for high-throughput testing and also for MS-imaging because they are connected by the fact that analyte desorption and ionization steps take place under ambient open-atmosphere conditions with no (or scarce) sample workup; yet there is no consensus on their classification.12 The primary ionization mechanism is the more frequently used classification criterion, breaking down ambient MS techniques into (i) those closely related to electrospray ionization (ESI) and (ii) those resembling atmospheric pressure chemical ionization (APCI), generally plasma-based techniques.13,14 Alternatively, ambient MS techniques may be organized by desorption or sample processing methods (i.e., thermal desorption, liquid extraction, use of lasers for desorption, etc.),15,16 and the combination of different criteria leads to establish subcategories. Readers interested in the fundamentals of different techniques and their classification according to the driving forces of both desorption and ionization steps are referred to different general reviews.17–25 More detailed information about a particular subcategory of ambient ionization techniques may be found in specific reviews on spray-based,26,27 plasma-based28–30 and laser-based methods.15,16
This review article is focused on the application of ambient MS to pesticide residue analysis in food and environmental samples. The review is broken down in two main sections: ESI-related and APCI-related ambient MS methods, providing an overview of different ambient desorption/ionization MS methods as well as representative examples of their application to pesticide residue determination.31,32 Different approaches, applied in the field, are presented, highlighting the advantages and limitations for their application in pesticide testing.
2. Electrospray-related ambient mass spectrometry methods applied to pesticide testing
In ESI-related ambient methods, analytes are desorbed from the sample, and transferred to the atmospheric pressure inlet of the MS as charged solvent microdroplets. An overview of more popular methods is presented in Fig. 2. Selected applications in pesticide residue analysis are summarized in Table 1.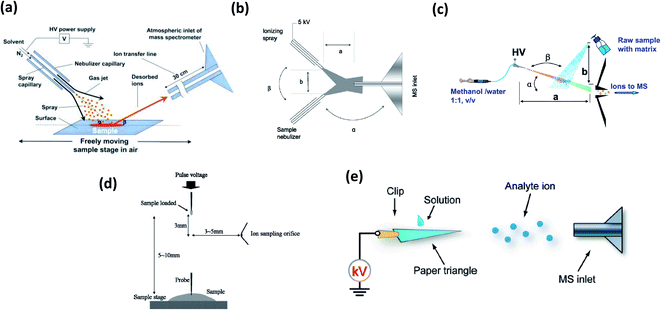 | ||
| Fig. 2 Schematic representations of (a) DESI (ref. 10); (b) EESI (ref. 45); (c) nanoEESI (ref. 49); (d) PESI (ref. 74); (e) PS-MS (ref. 59). For details, see text. Adapted with permission from the publishers (Wiley, Royal Society of Chemistry, ACS and AAAS). | ||
| Compounds | Matrix | Ambient ionization technique/spray solvent | Sample preparation | MS analysis | Analytical performance/matrix | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviations: ACN, acetonitrile; FA, formic acid; LCL, lowest calibration level; LIT, linear ion trap; LLE, liquid–liquid extraction; MS, mass spectrometry; QTRAP, hybrid triple quadrupole/ion trap; QqQ, triple quadrupole; TOF, time-of-flight. | ||||||
| DESI | ||||||
| 16 pesticides | (i) Fruit or vegetable extracts; (ii) fruit peels | DESI (4.5 kV, 5 μL min−1), ACN/water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (1% FA) 2) (1% FA) |
Two approaches: (i) QuEChERS; (ii) direct peel analysis | LIT-MS/MS | LODs 1–90 μg kg−1 in extracts | 36 |
| 17 pesticides | Fruits (mangoes, papaya, passion fruit, apples and strawberries), and honey | DESI (3.5 kV, 125 μm s−1), ACN/water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% FA) 1) (0.1% FA) |
Two approaches: (i) QuEChERS, and (ii) on the fruit surface | Orbitrap MS | LODs (pg mm−2): 1, on Teflon; 33 on apple peel | 37 |
| Chlorpropham | Potato surface | DESI (5 kV, 0.5 μL min−1), methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1% acetic acid) 1) (1% acetic acid) |
Not required | IT-MS/MS | LOD: 6.5 μg kg−1 | 38 |
| Dimethoate, tebuconazole, and trifloxystrobin | Olive and vine leaves | DESI (5.5 kV, 1 μL min−1), methanol/water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (10 mM FA) 2) (10 mM FA) |
Not required | QTRAP-MS/MS | LOQ (ng): dimethoate (50), tebuconazole (150), and trifloxystrobin (60) | 39 |
| Atrazine | Chinese cabbage leaf | DESI (4.5 kV, 4 μL min−1), ACN/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% FA) 1) (0.1% FA) |
Not required | IT-MS/MS | LOQ < 63.13 pg mm−2 | 40 |
| Dithiocarbamate fungicides: thiram and ziram | Fruit | DESI (5 kV, 5 μL min−1), methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% FA and 10 mM ammonium formate) 1) (0.1% FA and 10 mM ammonium formate) |
Surface extraction with acetonitrile | LIT-MS/MS | LCL: thiram, 1 mg kg−1; ziram, not detected | 41 |
| Alachlor, and atrazine and DEET | Leaf and vegetable surfaces | DESI, methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required | Portable IT-MS | 10 ng of DEET detected on the cornstalk leaf | 42 |
| Insecticides: dimethoate, imidacloprid, methiocarb, pyrethrins, and rapeseed oil | Plant stem and leaves | DESI-MSI (3.5–4 kV, 2.5–3 μL min−1), mixtures of methanol/water (containing FA, (NH4)HCO3 and NaHCO3) | Cryosectioning to study the pesticide incorporation into the plant | Orbitrap-MS | Not available | 44 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| EESI | ||||||
| Atrazine | Spiked urine (2 μL min−1) | EESI (5 kV, 5 μL min−1), methanol/water/acetic acid (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required | LIT-MS/MS | LOD: 0.4 fg atrazine | 46 |
| 204 toxicants, including 47 pesticides | Urine, blood, stomach content, liver | EESI (4 kV, 5 μL min−1), | Dilution and centrifugation (when needed) | LIT-MS/MS | LOD 0.002–0.09 μg L−1 | 47 |
| β-Cypermethrin and paraquat | Spiked farmland water | nanoEESI (5 kV, 0.05–0.1 μL min−1) with methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required | LIT-MS/MS | LODs (μg L−1):β-cypermethrin (6), and paraquat (10) | 48 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Paper spray (PS) and related methods | ||||||
| Atrazine, boscalid, clothianidin, diphenylamine, imidacloprid and thiamethoxam | Whole milk, olive oil, and leek homogenate | VeriSpray52 Paper Spray system (3.8 kV), methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9 water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% FA) 1) (0.1% FA) |
Not required/leek homogenization in a blender followed by solvent addition (1 mg μL−1) | QqQ-MS/MS | Calibration curves from the low μg L−1 (1–25) to the low mg L−1 (25–50) in milk and olive oil, and from 20 μg kg−1 to 10 mg kg−1 in leek homogenate | 53 |
| Methaldehyde | Environmental water | Paper spray (3.5 kV), methanol/water (0.1% FA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required. Only filtration | LIT-MS/MS | LOD: 0.05 μg L−1 | 54 |
| Atrazine, propazine, and metolachlor | Ground water, lake water, soil extracts, and crop extracts | Paper spray (3.5 kV), ACN | Soil and crop extracts by solid–liquid extraction with a mixture of acetonitrile/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) 20, v/v) |
IT-MS/MS | LODs in surface water: atrazine, 3.53 μg L−1; metolachlor, 1.70 μg L−1 | 55 |
| Aldicarb, imazalil, methiocarb, methomyl and thiabendazole | Oranges, grapefruits, lemons, limes, mandarins, tomatoes, apples, pears, strawberries, grapes, and sweet peppers | Paper spray (3.2 kV), H2O (0.1% FA)/ACN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
Two sampling approaches: (i) wiping with the paper; (ii) samples homogenized with acetonitrile | QqQ-MS/MS | LODs < 5 mg kg−1 in homogenates of orange, tomato and grapes | 56 |
| 36 pesticides | Red wine | Paper spray (3.5 kV), methanol or ACN | (i) Off-line QuEChERS; and (ii) on-line paper-adsorption | QqQ-MS/MS | LOQs: (i) 0.3–375.5 μg L−1 (23 analytes); (ii) ACN as spray solvent: 0.6–272.9 μg L−1 (34 analytes); MeOH as spray solvent: 0.9–280.4 μg L−1 (31 analytes) | 57 |
| Acephate, chlorpyrifos, and cyazofamid | Tomato peels | Paper spray (4.0 kV), methanol (0.1% FA) | Remove the peel of the tomato and perform an extraction with ACN, or water (acephate) | IT-MS/MS | LOQs: 30 ppb | 58 |
| Imazalil and thiabendazole | Peel of lemon | Paper spray (4.5 kV), methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required | LIT-MS/MS | Not available | 59 |
| Thiabendazole | Peel of treated oranges | Paper spray (4 kV), methanol/water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required (surface wiping) | Portable MS (ion trap) | Not available | 60 |
| Pyrazole fungicides: isopyrazam, fluxapyroxad, penflufen and pyraclostrobin | Spiked wines | Capillary paper spray (2.5 kV), methanol | Not required | QqQ-MS/MS | LOQs: 2 μg L−1 for each analyte | 61 |
| Atrazine and propazine | Spiked tap water | Paper spray with wax-printed channels (4.5 kV), methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required | Orbitrap-MS | LOD < 1.25 pmol | 64 |
| Acetochlor, alachlor, benzeneacetamide, butachlor, metolachlor, napropamid, and pretilachlor | Spiked milk | Paper spray with a silica-coated substrate, methanol/water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Not required | QqQ-MS/MS | LOQs: 10.9–242.4 μg L−1 | 67 |
| Herbicides: diuron and 2,4-dichlorophenoxyacetic acid (2,4-D) | Apples, bananas, and grapes | Paper spray with the MIP membrane substrate (3.5 kV), methanol (0.1% FA for positive ionization; 0.1% ammonium hydroxide for negative ionization) | Extraction with methanol | Q-Orbitrap-MS/MS | LLOQs: diuron, 0.41–0.99 μg L−1; 2,4-D, 1.02–2.0 μg L−1 | 68 |
| Carbofuran, methyl parathion, and parathion | Spiked orange surface (50 ppm) | Paper spray using paper coated with CNTs as the substrate (3 V), methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required (surface swabbing) | LIT-MS/MS | Not available | 69 |
| Atrazine, diuron and methomyl | Arugula, basil, cabbage, lettuce and kale vegetable samples | Paper spray with a paraffinned microchannel (3.5 kV), methanol (0.1% FA) | Extraction with methanol | Q-Orbitrap-MS | LOQs: 4.12–83.33 ppb | 65 |
| Atrazine, diuron and methomyl | Arugula, basil, cabbage, lettuce and kale vegetable samples | Leaf spray (3.5 kV), methanol (0.1% FA) | Not required | Q-Orbitrap-MS | LOQs: 0.11–120 ppb | 65 |
| Acetamiprid, diphenylamine, imazalil, linuron, and thiabendazole | Peel and pulp of different fruits and vegetables | Leaf spray (3.5 kV), isopropyl alcohol | Not required | LIT-MS/MS | LODs: 5–50 μg kg−1 | 71 |
| Beta-cypermethrin | Spiked apple juice | Wooden-tip ESI (3.5 kV), methanol (0.1% FA) | Not required | QTRAP-MS/MS | LOD: 30.0 μg L−1 (30.0 pg) | 73 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| PESI | ||||||
| Glufosinate and glyphosate | Human serum | PESI (1.7 kV), ammonium formate (10 mM)/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Dilution | QqQ-MS/MS | LOQs: 1560 μg L−1 for both herbicides | 76 |
| Paraquat | Human serum | PESI (1.7 kV), ammonium formate (10 mM)/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Dilution | QqQ-MS/MS | LCL: 15 μg L−1 | 77 |
| Acephate, acetamiprid, clothianidin, and thiophanate-methyl | Living plants | SF-PESI (2.5 kV), ACN/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% FA) 1) (0.1% FA) |
Not required | TOF-MS | LOD of acetamiprid in methanol solution over the Teflon substrate < 50 pg | 78 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| TD-ESI | ||||||
| 8 fungicides, 12 insecticides, and 2 herbicides | Fruits and vegetables | TD-ESI (4.5 kV), water (0.1% FA)/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Not required | QqQ-MS/MS | LOD: 0.5–100 μg L−1 for aqueous pesticide standards | 80 |
| 308 pesticides | Tomatoes and bell pepper | TD-ESI (4.5 kV), 5 mM NH4OAc in 40% MeOH | Not required | QqQ-MS/MS | LOD < 50 ppb, for benthiazole standard solution | 81 |
| Acephate, chlorpyrifos, diazinon, dimethoate, iprobenfos, and methamidophos | Gastric juice | TD-ESI (3.5–4.5 kV), water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% acetic acid) 1) (0.1% acetic acid) |
Not required | QqQ-MS/MS | LOD: 4.3–9.9 μg L−1 | 82 |
| Chlorpyrifos, dimethoate, methamidophos, methomyl, and paraquat | Human oral fluid | TD-ESI (3.5–4.5 kV), water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% acetic acid) 1) (0.1% acetic acid) |
Sampling with a cotton swab and subsequent pesticide extraction with methanol | QqQ-MS/MS | LODs: 1–10 μg L−1 | 83 |
2.1 Desorption electrospray ionization mass spectrometry (DESI-MS)
DESI was the first ambient ionization mass spectrometry method developed by Takáts and Cooks.10 It is commercially available.33 In the DESI experiment (Fig. 2a), a charged high-velocity spray of microdroplets is directed towards the sample (condensed-phase), and secondary droplets, including the species of interest, are then transferred through air to the atmospheric pressure interface of a mass spectrometer where solvent evaporation occurs, yielding gas-phase ionized compound(s). A solvent layer created by the initial spray dissolves the compounds deposited on the surface; subsequent spray droplets collide with the solvent layer, ejecting droplets containing the analyte from the surface towards the MS inlet.10 More detailed discussions on DESI operation can be found in selected specific reviews.34,35DESI has been applied to detect pesticides from both untreated crop surfaces and extracts obtained from dedicated sample workup procedures (e.g. QuEChERS (quick, easy, cheap, effective, rugged, and safe)).36,37 Representative agrochemicals including insecticides (e.g. isofenphos-methyl, malathion), herbicides (e.g. ametryn, atrazine), and fungicides (e.g. imazalil, prochloraz, triazoles) were detected at similar or lower concentrations than MRLs set. The use of isotopically labelled internal standards (ILIS) provided quantitative results in agreement with those obtained by reference methods using LC-MS/MS or GC-MS.36,37 DESI-MS fruit peel analysis was found to be a useful screening method to investigate samples containing pesticide residues, either analysing directly the fruit peel surface36 or by rubbing the peel with a glass slide subsequently used as a substrate for DESI-MS.37 Likewise, DESI-MS was used to determine chlorpropham on potato surfaces,38 dimethoate, tebuconazole, and trifloxystrobin on olive and vine leaves,39 and atrazine residues on Chinese cabbage leaves40 (see Table 1). The main limitations for quantitative analysis on surfaces are low precision and the presence of matrix effects.39
A challenging pesticide such as thiram could not be directly detected on the surface of pear leaves,41 but surface extraction with acetonitrile was appropriate for DESI-MS/MS. Using ILIS, semi-quantitative results could be demonstrated in spiked samples. Extraction of the homogenized fruit by the QuEChERS method was inappropriate, due to severe suppression effects.
The feasibility of high-throughput in situ screening methods would be a convenient and cost-effective approach, as the number of samples subjected to a comprehensive evaluation would be significantly reduced. The combined use of ambient ionization methods and portable (handheld) mass spectrometers represents an interesting option to move the food control from the laboratory to the market shelves. This was first demonstrated by Mulligan et al.42 using DESI to detect DEET, alachlor and atrazine in leaves and vegetable surfaces with no sample treatment. Sensitivity, selectivity and rapid detection could be satisfactorily achieved for the detection of target compounds in relevant fields of analysis. Thus, DEET on the surfaces of cornstalk leaves or tomatoes was detected below 10 ng.
The implementation of ESI-related ionization sources in portable mass spectrometers for in situ analysis of real samples is a very interesting approach that has been explored for pesticide residue testing, using not only DESI but also PS techniques.42,43 Another interesting feature explored with DESI is the development of chemical images (mass spectrometry imaging (MSI)) of pesticide residues in crops (DESI-MSI). The distribution of pesticides in different parts of Cotoneaster horizontalis and Kalanchoe blossfeldiana was investigated by Gerbig et al.44 using DESI-MSI. The distribution of contact pesticides (pyrethrins, rapeseed oil, imidacloprid, and methiocarb) on the plant surface and the redistribution of a systemic pesticide (dimethoate) in the plant stem and leaves were analyzed by DESI-MSI. A mass range from m/z 300 to 1500 was acquired to detect pyrethrins and triglyceride (TG) ions present in rapeseed oil. The TG showed non-homogeneous covering on the leaf surface. Also, pyrethrins showed different distributions on the leaf surface. This was due to differences in polarity and size between them. When the same experiment was performed using an insecticide which contained imidacloprid and methiocarb, pesticide distributions found on the leaf were distinctly more homogeneous. The systemic incorporation of dimethoate in a Kalanchoe blossfeldiana plant was studied, and dimethoate was detected in the transport system of the plant after 25 days of treatment, and it was found to be homogeneously distributed in a leaf section after 60 days.
2.2 Extractive electrospray ionization (EESI) and nanoEESI
EESI was introduced for the first time by Chen et al.45 (Fig. 2b). It is based on the use of two separate sprayers, one of them (1–10 μL min−1) nebulizes the sample solution and the second one generates charged microdroplets of solvent (extractive ESI) which continuously extracts analytes from the sample solution into the solvent spray. It can also be modified to allow the analysis of solid samples (through neutral desorption EESI) or gas phase samples. This technique is very interesting for fast analysis of complex samples, being able to detect traces of atrazine in raw urine,45,46 and of more than 200 toxicants, including pesticides, in urine, blood and stomach content or liver samples.47Nanoextractive electrospray ionization (nanoEESI)48 mass spectrometry is based on the use of a nanospray to generate microdroplets (Fig. 2c), using solvent flows in the range of 0.05–0.1 μL min−1. This avoids the use of the sheath gas, thus reducing the parameters needing optimization and allowing hyphenation to portable mass spectrometers. Sample contamination and memory effects are reduced by using a disposable and manual sample injector. NanoEESI was applied to ambient mass analysis of paraquat and β-cypermethrin in spiked farmland water.48
2.3 Paper spray and related methods
In paper spray (PS),49 a piece of paper ending with a sharp point (held in front of the mass spectrometer inlet) is wetted with a solvent; a high electric field is applied and the capillary action allows analyte transport and ionization (Fig. 2e). Samples are loaded onto the paper by direct addition (a volume below 10 μL is appropriate), or the paper can be used as a swab to sampling surfaces. The solvent is then applied once and mass spectra are recorded continuously until the signal disappears. With regard to the actual mechanisms, according to Espy et al.,50 two spray operation modes have been described in positive ion PS-MS, spray mode 1, and spray mode 2 – after significant solvent depletion. In the first mode, multiple Taylor-cone jets are observed, which depends on the paper cut and the solvent composition with ions from proton transfer reactions dominating the mass spectra. In spray mode 2, a single cone-jet and a corona discharge coincide, with electron-transfer ions and radicals being observed (it is supposed that mode 2 occurs always in negative ion MS).Although most PS applications have been performed with in-house built setups,51 commercial devices based on PS ionization (e.g. VeriSpray™ PaperSpray ion source) are available and have been tested for pesticide analysis in whole milk, olive oil and leek homogenate.52,53 PS has been used for the determination of methaldehyde (molluscicide)54 and herbicides55 in environmental waters by direct addition of a sample aliquot onto the paper substrate. Acidification of the solvent favored the formation of protonated molecules against sodium adduct, thus lowering LODs.54 The use of an isotopically labeled IS allowed the quantification of atrazine and metolachlor in the low microgram per liter range by PS-MS/MS. Complex matrices, such as soil extract55 or fruit homogenates,55,56 showed higher limits of detection. In contrast, Guo et al.57 reported that wine samples directly applied on the paper substrate allowed better detection and quantification (using ILIS) than when QuEChERS extracts were prepared. In a recent study, a semi-quantitative approach based on the extraction of tomato peels, instead of the whole vegetable, allowed us to distinguish between stored or field samples.58
For screening purposes, PS-MS/MS allowed the detection of fungicides present on real samples by swabbing fruit peel with paper wetted with solvent, which is further used as a substrate.43,59 The spectra obtained for some citrus fruits showed the presence of imazalil and thiabendazole, identified by MS/MS analysis.59 Sampling by paper wiping has the advantage of collecting a larger amount of analyte from a larger surface area, so higher intensities may be obtained compared to surface analysis of agrochemicals by other ambient techniques such as DESI or LTP.59
PS has been combined with portable mass spectrometers to perform “in situ” analyses, including pesticide testing in food surfaces.43,60 Soparawalla et al.60 determined thiabendazole by PS-MS in oranges using commercially available lens wipes paper (pre-moistened with isopropyl alcohol) on the sample orange surface and as an ionization substrate. Nevertheless, signals, as well as their duration, were one third lower than those obtained from filter paper, which was explained as a consequence of the different porosity of both paper substrates.60 Indeed, the substrate plays a key role in PS-MS, and although both filter55,60 and chromatographic paper49,59 have been widely used, many modifications in the composition of the substrate have been proposed and are described as follows.
The use of a capillary emitter embedded on the paper substrate showed a positive influence on the sensitivity and reproducibility compared with standard PS.61 Pu et al.62 developed a method for the detection of pyrazole fungicides (penflufen, isopyrazam, fluxapyroxad, and pyraclostrobin) in wine using this PS variation with 10 μL of sample with no treatment and bixafen as the IS. LOQs of 2 ng mL−1 were obtained, in compliance with the required regulatory limits. Microfluidics technologies, such as photolithography and wax patterning, have also been tested in order to increase sensitivity.63,64 Photolithography produced a high background signal, but wax barriers improved sensitivity in the detection of atrazine and propazine in spiked tap water, compared to standard PS.63 Paraffin microchannels also showed good results in pesticide analysis (atrazine, diuron and methomyl) in vegetable extracts.65
Chemical modification of substrates was also tested. For instance, a urea-modified paper substrate improved sensitivity in negative PS-MS because it retained anions from the sample solution, thus reducing adduct formation.66 In positive ion mode, a silica-coated paper substrate improved LOQs for 7 pesticides in milk compared to the commercial paper substrate.67 Molecularly imprinted polymers (MIP) have also been combined with PS ionization for herbicide analysis in food.68 MIPs were directly synthesized on cellulose membranes, which were loaded with samples by dipping in different fruit methanolic extracts (apples, bananas and grapes), and then were used as PS substrates after washing and drying. Remarkable selectivity and LOQs below the established MRL (100 μg L−1) were achieved for diuron and 2,4-dichlorophenoxyacetic acid (2,4-D), in positive and negative ion modes, respectively.68 It is also worth mentioning the use of substrate paper coated with carbon nanotubes (CNTs)69 which enabled the ionization of pesticides on orange peel with low voltages – in the range of volts instead of kilovolts commonly used in PS-MS.
Finally, a smart and environmentally friendly modification of PS consists of the replacement of the paper with a natural porous substrate, the sample itself. In this regard, leaf spray (LS)70 is a variation of the PS where the plant tissue acts simultaneously as a substrate, sample and ion source. In this method, the gas phase ions are generated directly from the plant tissue, no other ionization device or support is needed beside the application of HV and a solvent. The direct determination of agrochemicals in fruit and vegetable tissues with no sample pre-treatment was demonstrated.65,71 Signals were observed even without solvent addition, due to the presence of natural juice on fruit and vegetables, but more intense signals and better signal to noise ratios were obtained by adding solvent.71 LODs below EU MRLs were reported65,71 and discrimination between organic and conventional samples was shown,71 also providing a semi-quantitative estimation of the concentration of pesticides in non-organic samples by external calibration. Another variation of paper spray for pesticide analysis is the wooden-tip ESI,72 in which the porous substrate is a toothpick. The narrow-stick shape allows the generation of sharp electrosprays. Sample loading can be carried out by pipetting or directly dipping the wooden-tip into the sample solution. When a high voltage is applied and a few microliters of solvent are added to the tip, spray generation takes place and analyte ions are transferred to the MS. Analysis of beta-cypermethrin in spiked apple juices was satisfactorily performed as a proof of principle of this approach.73
2.4 Other electrospray-based ionization methods: PESI and TD-EDI
A solid needle electrospray probe for liquid sample analysis called probe electrospray ionization (PESI) (Fig. 2d) was developed by Hiraoka et al.74 A small amount of liquid sample is picked by the needle, with an automated movement on the vertical axis. Then, the needle is positioned in front of the MS inlet and an applied HV leads to ESI of the sample. PESI is free from clogging problems compared to ESI-based ion sources using capillaries. This source, commercially available,75 has been applied to the determination of polar pesticides (glufosinate and paraquat) in human serum from real poisoning cases76,77 with results consistent (using IS) with those obtained by standard methods. A variant (sheath-flow probe electrospray ionization (SF-PESI))78 using a sheath liquid flow with a solid probe was applied to pesticide analysis in real-time from living plant tissues. Acephate, acetamiprid and thiophanate-methyl applied to the plant were detected, finding intense signals of sodium and potassium adducts together with the protonated molecule. However, the presence of these adducts and the lack of reproducibility in the sample amount loaded in the needle probe prevent SF-PESI from providing absolute quantification values.A relatively similar approach was proposed by Shiea et al., so called thermal desorption electrospray ionization (TD-ESI-MS).79 A metal probe is used to sample analytes; then, the probe is located in a pre-heated oven (Fig. 3), with analytes being desorbed with a nitrogen gas stream, transferred into an ESI plume to be ionized, and subsequently detected by MS.
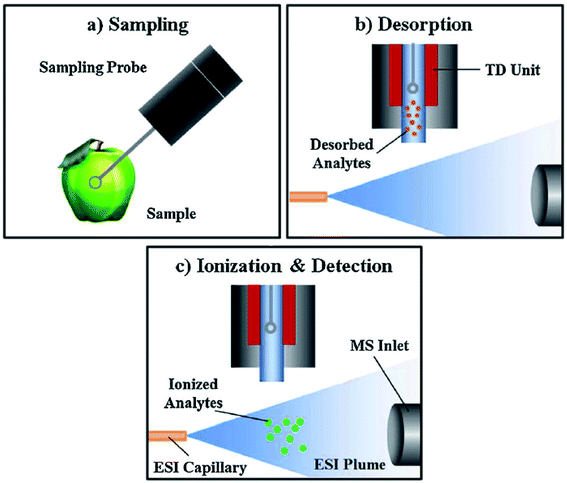 | ||
| Fig. 3 Schematic diagram of the TD-ESI-MS analytical procedure. Adapted from ref. 83, with permission from Wiley. | ||
TD-ESI has been used to detect pesticide residues from the surfaces of fruits and vegetables.80,81 The decay, distribution, and removal of pesticides from fruit and vegetable surfaces by soaking in water or detergent baths were studied.80 The technique was useful for the screening of pesticides, but quantitative results could not be provided by TD-ESI in solid samples due to the inhomogeneous distribution of analytes throughout the surface.80,81 TD-ESI has also been applied in the forensic field for the rapid identification of ingested pesticides.82,83 A set of pesticides commonly detected in self-poisoning patients in Taiwan have been analysed by TD-ESI in gastric juice and oral fluid, achieving LODs at the parts per billion level (see Table 1). This involves a quick analytical process, which allows the rapid identification of pesticides before they reach the blood stream in self-poisoning patients, thus offering a promising tool for point-of-care based on ambient mass spectrometry.
3. Atmospheric pressure chemical ionization (APCI)-related ambient mass spectrometry methods applied to pesticide testing
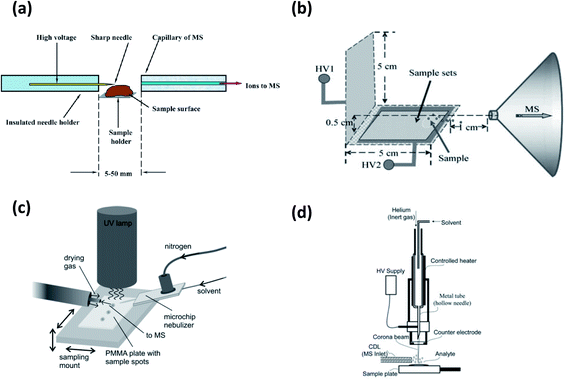
APCI-related ambient MS methods include those which use an electric discharge to generate the species responsible for analyte ionization. Analyte ions are formed through a series of gas-phase ion–molecule reactions with environmental reagent species produced by a type of discharge. Additionally, in plasma-based techniques, ionization is also produced by energy-transfer reactions between the activated reagent species (e.g., helium metastables) and analyte molecules.14 Positive ionization is mainly attributed to Penning ionization and proton transfer from water cluster ions, whereas a variety of mechanisms such as electron capture and anion attachment have been proposed for negative ionization.84 This versatility of mechanisms allows the ionization of species within a wider range of polarities than ESI-based methods. Nonpolar compounds, such as organochlorinated pesticides, PAHs or polybrominated diphenyl ethers (PBDEs), which are often associated with GC-MS with electron impact ionization or chemical ionization (vacuum) can be effectively ionized with the methods described as follows. This feature makes plasma-based methods very useful for nontargeted or unknown studies given the different ionization mechanisms that apply at the same time.In these ionization sources, a gas flow (e.g. He, N2 or air) is excited by an electrical discharge produced between two electrodes by applying either a direct-current (DC) or an alternating-current (AC) voltage at frequencies from kilohertz to several megahertz. Here, APCI-related ionization sources are sorted out into three groups, according to the featured discharge: (a) glow discharge (GD) which is generated by DC voltage currents from hundreds of microamperes to several milliamperes and heating of the plasma gas; (b) corona discharge (CD) which is produced around the tip of a needle electrode by DC supply and generates currents in the low microampere range; and (c) dielectric barrier discharge (DBD) which is generated by an AC supply between two electrodes separated for at least one dielectric layer, providing a plasma close to room temperature and currents in the microampere range. For details about the fundamentals of plasma physics the readers are referred to specific literature.85 Schematic representations of APCI-related ionization sources are shown in Fig. 4 and 5. A summary of different methods developed for pesticide analysis using these sources is shown in Table 2.87–141
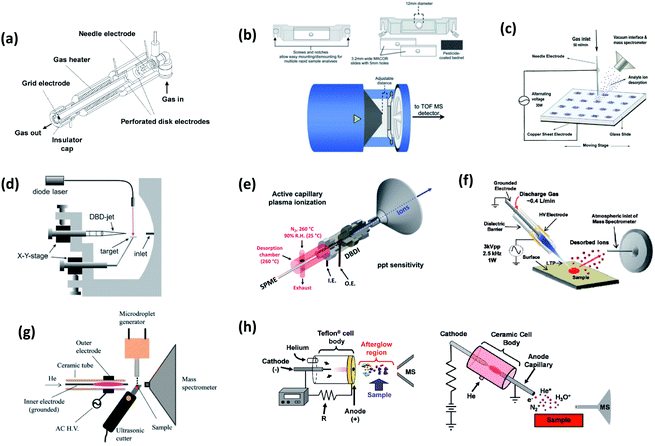 | ||
| Fig. 4 Schematic representations of (a) DART (ref. 11); (b) sample holder and upper view of TM-DART (ref. 86); (c) DBDI (pin-to-plate) (ref. 121); (d) LD-DBDI (ref. 123); (e) ACaPI source (inner (I.E.) and outer electrodes (O.E.) are shown) coupled to the SPME desorption chamber (ref. 128); (f) LTP (ref. 118); (g) ultrasonic-assisted desorption DBDI-MS (ref. 136); (h) pin-to-plate and pin-to-capillary configurations of FAPA (ref. 103). For details, see text. Adapted with permission from the publishers (ACS, Elsevier and Royal Society of Chemistry). | ||
 | ||
| Fig. 5 Schematic representations of: (a) DAPCI (ref. 110); (b) TDCI (ref. 111); (c) DAPPI (ref. 138); (d) DCBI (ref. 113). For details, see text. Adapted with permission from the publishers (Wiley, Elsevier, Royal Society of Chemistry and ACS respectively). | ||
| Compounds | Matrix | Ambient ionization technique | Sample preparation | MS analysis | Analytical performance | Ref. |
|---|---|---|---|---|---|---|
| a IT, ion trap; IT-SPME, in-tube solid phase microextraction; LIT, linear ion trap; Q, single quadrupole; QqQ, triple quadrupole; Q-TOF, quadrupole time-of-flight. | ||||||
| DART | ||||||
| Strobilurins fungicides: Azoxystrobin, dimoxystrobin, kresoxim-methyl, picoxystrobin, pyraclostrobin, and trifloxystrobin | Wheat | DART, He; confirmatory analysis: DESI, methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.1% formic acid) 1) (0.1% formic acid) |
DART-TOFMS: extraction with ethyl acetate. DESI-MS/MS: microextraction with a C18 pipet tip using methanol as the extracting solvent | TOFMS | LOQs 5–30 μg kg−1 | 87 |
| Dicyandiamide | Powdered milk | TM-DART using He as the ionization gas | Extraction with acetonitrile/water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Q-TOFMS | LOQ: 250 μg kg−1 | 88 |
| 50 pesticides (positive and negative ionization) | Red and white wine | TM-DART, He | Modified QuEChERS procedure | QqQ-MS/MS and TOFMS | (i) DART-QqQ MS/MS, LOQs 1–100 μg L−1; (ii) DART-TOFMS, LOQs 25–250 μg L−1 | 89 |
| 31 pesticides (positive ionization) | Red and white wine | TM-DART, He | Direct determination | QqQ-MS/MS and TOFMS | (i) DART-QqQ MS/MS, LOQs 25–500 μg L−1; (ii) DART-TOFMS, LOQs 100–500 μg L−1 | 90 |
| Amitrole, cyromazine, diethanolamine, melamine, propamocarb, 1,2,4-triazole, and triethanolamine | Lettuce and celery | DART using He as the ionization gas | Modified QuPPe procedure | Orbitrap MS | LOQs 50–190 μg kg−1 | 91 |
| Thiram and ziram | Fruits | DART, He | Surface extraction with acetonitrile | TOFMS and Orbitrap MS | LOQs: (i) DART-TOF: 1000 and 500 μg kg−1 for thiram and ziram, respectively; and (ii) DART-Orbitrap: 100 and 1000 μg kg−1 for thiram and ziram, respectively | 41 |
| 132 pesticides | Surfaces of grapes, apples, and oranges | TM-DART using He as the ionization gas | Sampling with foam swabs | Orbitrap MS | Using foam swabs: 86% target analytes detected at levels of 2 μg kg−1 (apples or oranges) and 10 μg kg−1 (grapes) | 93 |
| Mixtures of 240, 140, 132 and 60 pesticides | Surfaces of apples, kiwis, peaches and tomatoes | DART using He as the ionization gas | Sampling with foam swabs | Orbitrap MS | More than 80% of target analytes were detected at levels of 2, 5 and 10 μg kg−1 | 94 |
| 164 pesticides | Surfaces of apples, oranges, and broccoli | TM-DART using He as the ionization gas | Sampling with polyurethane foam disks | Q-Orbitrap MS | Spiked pesticides at a concentration of 10 μg kg−1 were 100% detected on apples and oranges, and 80% on broccoli | 95 |
| Dimethoate, malathion, and methamidophos | Surfaces of cherry tomatoes, oranges, peaches, and carrots | TM-DART using He as the ionization gas | Sampling by two kinds of swabs: (i) cotton, and (ii) polyester | Orbitrap MS | Concentrations (μg L−1): dimethoate (20 and 200), methamidophos (200), and malathion (80 and 800) | 96 |
| Ametryn, atrazine, prometon, prometryne, propazine, and simazine | Lake water and orange juice | DART using N2 as the ionization gas | IT-SPME | TOFMS | LOQs: 0.06–0.46 μg L−1 | 97 |
| 19 pesticides | Concord grape juice, orange juice, cow milk, and river water | TM-DART using He as the ionization gas | SPME extraction performed on the coated mesh | QqQ-MS/MS | LOQs, concord grape juice and surface water (0.1–5 μg kg−1); orange juice (0.1–0.5 μg kg−1); cow milk (0.1–1 μg kg−1) | 99 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| APGD | ||||||
| Thiabendazole | Lemon skin | FAPA (pin-to-plate design), He gas flow, 0.8 L min−1; operating current, 25 mA | Surface rubbed with a polyester swab | TOFMS | Not available | 101 |
| 10 pesticides | Spiked fruit juices and fruit peel (apples, cranberries, grapes, oranges, and salad leaves) | FAPA (pin-to-plate design), He; DC 0.5 kV, 40 mA | Fruit juices spotted on filter paper; pesticide solution spotted on the fruit/vegetable surface | Q-TOF-MS/MS | LODs, (i) fruit juices, 1–500 μg L−1; (ii) apple skin, 0.01–5 μg kg−1 | 102 |
| Ametryn, diphenylamine, ethoxyquin, isofenphos-methyl, isoproturon, malathion, parathion-ethyl, and terbuthylazine | Standards | FAPA (pin-to-capillary design), He, | Not required | LIT-MS/MS | LODs: 0.004–9.2 fmol | 103 |
| Ametryn, amitraz, buprofezin, dimethoate, diphenylamine, imazalil, isoproturon, malathion, and parathion-ethyl | Peels and extracts of apples, oranges, tomatoes, green peppers, grapes, and celery | MFGDP, He 0.6 L min−1; DC 820 V, 15 mA | Two approaches: (i) QuEChERS, and (ii) direct determination on peel | IT-MS/MS | LODs, 0.13–3.09 μg kg−1 for fruit and vegetable extracts (QuEChERS) | 105 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Corona discharge | ||||||
| Atrazine | Unripe pumpkin surface and cloths | DAPCI using ambient air as the discharge gas | Not required | LIT-MS/MS | 1–10 pg of atrazine detected | 110 |
| Dimethoate | Orange juices | TDCI using ionic liquid (1-butyl-3-methylimidazolium bromide salt) | Not required | LIT-MS/MS | LOD 0.9 ng L−1 | 112 |
| 12 pesticides | Standards | DCBI source using He as the discharge gas | Not required | Q-MS | LODs 1–9.6 ng | 113 |
| Acephate, isoprocarb, dimethoate, dichlorvos, and dicofol | Spiked water, river water, tap water, and wastewater | DCBI source using He as the discharge gas | Microextraction in the PDMS substrate | LODs of 1 μg L−1 | 114 | |
| Dicrotophos, pirimicarb, carbaryl, and triazophos | Standards | RTILs matrix-assisted DCBI, using He as the discharge gas | Not required | Q-MS | LODs: 2–10 ng | 115 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| DBD | ||||||
| Prochloraz, propazine, and spinosad | Standards | LD-DBDI, He 0.2 L min−1; AC, 20 kHz, 4.5 kV | None | ITMS | Not available | 123 |
| Dichlorvos, diethyl ethylphosphonate, diisopropyl methylphosphonate, dimethyl methylphosphonate, and diethyl phosphoramidate | Standards | ACaPI, 1.6 kV, 5.75 kHz | None | Portable LIT-MS/MS | LODs: 1.0–6.3 μg L−1 | 124 |
| 13 agrochemicals | Fruit peels, fruit/vegetable extracts and water | LTP, He 0.4 L min−1; AC, 2.5 kHz, 5–10 kV; 150 °C heated surface (except direct peel analysis) | Two approaches: (i) direct determination on fruit peel or spiked water, and (ii) QuEChERS extracts | LIT MS/MS | LODs QuEChERS extracts: pepper (0.4–200 μg kg−1); oranges (0.4–20 μg kg−1); tomatoes (0.2–20 μg kg−1) | 132 |
| Acetamiprid, cyprodinil, fenhexamid, and fludioxonil | Grapes and raspberries | LTP, He 0.3 L min−1; AC, 10 kV, 30 kHz; 150 °C heated substrate | QuEChERS (citrate buffer) extracts | Orbitrap MS | LOQs ranged from 1 to 70 μg kg−1 | 133 |
| 10 multiclass fungicides | Red wine | LTP, He 0.45 L min−1; AC, 6.2 kV, 2.5 kHz; 120 °C heated substrate | Dilution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) with ACN 5) with ACN |
IT-MS/MS | LODs, ranged between 15 and 300 μg L−1 | 134 |
| 12 pesticides | Broomcorn | TD-LTP, He 0.15 L min−1; 180 °C for TD; 0.1 L min−1 air flow for sample transport | Methanolic extraction in an ultrasonic bath | QqQ MS | LODs ranged between 10 and 1000 μg L−1 | 135 |
| Carbaryl, gramicidin S, imazalil, and spinosad | Standards | Modified LTP, He 0.25 L min−1; desorption by an ultrasonically vibrating blade | None | Orbitrap MS | LODs 0.1–100 ng | 136 |
| Diphenylamine | Apples | LTP (reduced size), He 0.3 L min−1; AC, 17 kV, 6 kHz | None | LIT-MS/MS (portable) | — | 60 |
| 11 pesticides | Standards | Handheld LTP, 7.4 V, 900 mA h Li–polymer battery; (i) air, 0.1 L min−1; (ii) and (iii) He, 0.1 L min−1 | None | (i) And (ii) LIT MS/MS; (iii) mini MS | (i) LTP (air)-MS/MS, LODs 0.004–300 ng; (ii) LTP (He)-MS/MS, LODs 0.001–0.9 ng; (iii) LTP (He)-MS (mini), LODs 0.1–200 ng | 137 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Photoionization | ||||||
| Aldicarb, carbofuran, ditalimfos, imazalil, methiocarb, methomyl, oxamyl, pirimicarb, and thiabendazole | Standards and orange peel | DAPPI using different solvents (acetone, toluene, and anisole) as spray solvents. For orange peel analysis, acetone was selected as the spray solvent | Not required | IT-MS | LODs: 30–300 pg (0.14 to 1.4 pmol) | 140 |
| Acetamiprid, clothianidin, imidacloprid, thiacloprid, and thiamethoxam | Standards and thiacloprid detection on fresh rose leaves and turnip rape flowers | DAPPI using acetone as the spray solvent | Not required | IT-MS | LODs: 0.1–1 μg L−1 (0.4–5.0 fmol) for standard analytes | 141 |
3.1 Plasma sources based on an atmospheric pressure glow discharge (APGD)
A set of different sampling assemblies have also been developed together with DART including the, so called, transmission mode DART (TM-DART),86 and different autosamplers and pipette-based devices for sampling solid, liquid or gas samples. Thus, strobilurin fungicide residues were determined in wheat samples by Schurek et al.87 by DART-TOF-MS. The utility of the DART-TOFMS method for a rapid qualitative screening of the target fungicides in wheat grains without sample preparation requirements was attempted at concentration levels close or higher than the established MRLs. For quantitative purposes, the extraction of pesticides was carried out with ethyl acetate prior to DART-TOFMS analysis. The obtained LOQs (ranging 5 to 30 μg kg−1) were lower than MRLs, and approached the results obtained by conventional LC-MS/MS using QuEChERS extraction.
The same group also showed the applicability of this methodology for the analysis of two dithiocarbamate fungicides (thiram and ziram) in pears.41 Solvent extraction of the fruit surface with acetonitrile was preferred to the QuEChERS procedure. The obtained LOQs comply with the EU-MRLs of fruit crops, and quantitative analysis was possible using ILIS. These results were compared to surface analysis of fruits by DESI-MS/MS, which was suitable for thiram but not for ziram.
DART-Q-TOF-MS/MS was used in a collaborative study of Zhang and Dong88 for the confirmation and quantification of dicyandiamide in powdered milk, using simple extraction with a mixture of water and acetonitrile. Quantitative analyses were performed with a high reproducibility without ILIS (commonly used to correct fluctuations in the desorption step) using TM-DART (Fig. 4b). The results showed that TM-DART was useful for semi-quantitative analysis of pesticides in insecticide-treated nets at concentration levels lower than 0.5 mg m−2 (10 ng) of deltamethrin, using either He or N2 as the discharge gas. The use of a fixed geometry eliminated the need for sample position optimization.
Zhang and Dong also reported that TM-DART provided enhanced precision compared to other sampling devices for the determination of pesticide residues in wine samples.89,90 Quantitative analysis of the targeted pesticides was performed with a triple quadrupole instrument operated in multiple reaction monitoring mode. Direct determination of pesticides in red or white wine was achieved in 3 min with LOQs ranging from 25 to 500 ng mL−1. However, QuEChERS treatment was found to be useful to minimize matrix effects and improve sensitivity (for 31 out of 50 pesticides) and LOQs (decreased up to 1–100 ng mL−1).90 Likewise, Lara et al.91 also implemented the use of the Quick Polar Pesticide extraction (QuPPe) method92 with an additional clean up step in order to enable the determination of a group of polar pesticides in lettuce and celery by DART coupled to HRMS.
The use of foam swabs wetted with a solvent as the sampling method on the surfaces of fruits and vegetables has been tested. Polyurethane foam swabs were proven to be effective for the analysis of pesticide mixtures containing over two hundred species.93–95 A temperature gradient in the DART gas heater allowed the detection of such a great number of pesticides in a 3 min run. Cotton and polyester cleaning swabs were also useful96 although polyester swabs have the disadvantage that their “background” ions themselves dominate the spectrum.
Desorption temperature provided by the gas heater is one of the most critical analyte-dependent factors to be optimized, since it must be compatible with the sampling method (i.e. not degrading swabs or solid substrates) while providing effective desorption with a high signal (which may include thermally labile compounds).
Solid-phase microextraction (SPME) has been combined with DART for analyte preconcentration and reduction of matrix effects for liquid samples. Wang et al.97 analyzed triazine herbicides in lake water and orange juice by coupling of in-tube SPME (IT-SPME) with DART-MS. IT-SPME is based on the use of a carbon-nanotube-incorporated polymer monolith and the online analyte desorption by the DART-MS system, leading to analyte desorption and ionization. Method precision was improved using an ILIS, and LOQs ranged from 0.06 to 0.46 ng mL−1. The direct hyphenation of SPME to TM-DART (SPME-TM-DART) was introduced by Gómez-Ríos and Pawliszyn,98 based on a metallic mesh coated with adsorbent particles which extracts the target analytes. SPME-TM DART devices were used for screening and quantification of pesticides in food (grape juice, orange juice, and cow milk) and environmental matrices (river water).99 The total analysis time did not exceed 2 min per sample with LOQs in the range of 0.1–5 μg kg−1.
Therefore, to some extent, the analysis of pesticide residues in complex samples by DART-MS, and also by most of the ambient MS methods described, requires some sample treatment in order to reduce matrix effects to achieve both LOQs complying with the stringent regulations and to improve precision. Notably, some approaches that utilize minimal sample manipulation (e.g. surface extraction, SPME) give satisfactory quantitative results, particularly when ILIS is used.
For instance, thiabendazole was detected on lemon skin by wiping the surface with a swab and exposing it to FAPA.101 The direct exposure of apple skin spiked with a mixture of pesticides (alachlor, atrazine, carbendazim, carbofuran, dinoseb, isoproturon, metolachlor, metolcarb, propoxur, and simazine) yielded LODs in the range of 0.01–2.0 ng g−1, below the MRLs set by the EU for the entire crop (in the range of 50–100 ng g−1).102 Trace analysis of pesticides in spiked fruit juices from apples, cranberries, grapes, and oranges was performed by pipetting 1 μL of the juice into pieces of filter paper subsequently exposed to the afterglow. A hybrid Q-TOF was used, but the response of spiked fruit juices at different concentrations was not linear and the precision was around 20%. A standardized method for sample positioning, together with the use of ILIS may solve the problems associated with reproducibility.
Shelley et al.103 developed an improved design of the FAPA source (Fig. 4h) leading to background signal reduction in both positive and negative ionization modes (89% and 99%, respectively), and, in addition, the capillary anode reduced the quantity of atomic oxygen (responsible for analyte oxidation in the pin-to-plate configuration). LODs obtained were ca. one order of magnitude better than related plasma-based methods.
An approach, conceptually similar to FAPA, that has also been reported for pesticide testing, is microfabricated glow discharge plasma (MFGDP). It consists of a small planar ceramic chamber with DC voltage applied between two plate electrodes.104 It features lower gas temperatures than FAPA. Semi-quantitative analysis of pesticides was performed with QuEChERS extracts of fruits and vegetables by MFGDP-MS/MS.105 The solutions were spotted onto a filter paper and exposed to the plasma, achieving LODs between 0.13 and 3.1 ng g−1 and linearity up to two orders of magnitude.
3.2 Ambient mass spectrometry methods based on corona discharge ionization
Amongst these methods, Desorption Atmospheric Pressure Chemical Ionization (DAPCI)106,107 (Fig. 5a) is based on the same principle as APCI. A corona discharge is generated on the tip of the sharp needle (by applying a DC voltage of a few kV), and reagent species are subsequently generated in its surrounding environment. Both gases and liquid solvents (introduced through an evaporation chamber) may be used to form reagent ions. DAPCI has shown excellent results for the detection of different drugs and biological samples,108,109 with signal improvement compared with both DART and DESI. As an example, signals for cinchocaine and hydrocortisone (the main ingredients of an analysed ointment) were 5 and 50-fold higher with DAPCI than with DESI.108 Chen et al.110 used DAPCI to detect picograms of atrazine directly on an unripe pumpkin surface and in cloths. MS/MS analyses together with chlorine isotope patterns were used to confirm the presence of the herbicide.Besides DAPCI, other corona-based ambient MS methods are also shown in Fig. 5. Thermal dissociation atmospheric chemical ionization (TDCI) was developed in 2011 by Han et al.111 Ionic liquids (green solvents) are used to produce reagent ions by thermal dissociation processes; these reagent ions interact with the analytes of the raw samples yielding analyte ions that are transferred to the MS. The original design of this source included two electrode plates assembled in a 90-degree configuration in front of the MS inlet and a heatable sample holder (see Fig. 5b). The detection of both polar and nonpolar (nonvolatile) compounds was demonstrated.111 A second design was used by Ouyang et al.112 for dimethoate in orange juices, achieving a low LOQ (0.9 pg mL−1) with no sample workup. Nevertheless, the authors reported some constraints of the technique due to the use of ILs, such as the possible contamination of the ion source with the continued used of these solvents and their high proton affinity, which hinders its application.
Another corona-based approach described by Wang et al.113 is desorption corona beam ionization (DCBI) (Fig. 5d). It has similarities with the DART source, such as the use of He (discharge gas) and the need of heating the gas for sample desorption. The DCBI source produces a visible corona beam, allowing sampling area localization, thus being useful for imaging/surface experiments. In addition, it also allows gradient temperature operation, which permits sequential sample desorption to achieve a rough separation of analytes from complex mixtures. Pesticides were studied using this source, achieving absolute LODs ranging between 1 and 9.6 ng. In order to avoid sampling difficulties in liquid or gaseous matrices, the use of polydimethylsiloxane (PDMS) was also proposed as a sampling substrate (by immersion in water).114 An improvement in LODs (1 μg L−1) for pesticide (acephate, isoprocarb, dimethoate, dichlorvos, and dicofol) detection in water, together with an increase in the number of identifiable compounds was achieved. Likewise, other improvements were proposed by Wang et al.115 based on room temperature ionic liquid (RTIL) matrix-assisted DCBI.
3.3 Ambient mass spectrometry methods based on dielectric barrier discharge
Dielectric barrier discharges (DBDs) are widely used for plasma generation, because they offer some attractive features such as stable operation at atmospheric pressure, small size, low power consumption and cold plasma production.28 Several designs of DBDs have been proposed for ambient MS including one or two dielectric barriers between the electrodes.116,117 Amongst them, low-temperature plasma (LTP)118 and the so-called DBDI119,120 have been used and compared for pesticide residue testing. LTP is based on a ring-to-pin configuration and one dielectric barrier, whereas DBDI is based on a ring-to-ring configuration. Na et al.121 reported the first ambient DBDI source (Fig. 4c). It was a pin to plate configuration composed of a discharge needle (a hollow stainless-steel needle) and a copper sheet electrode, both separated by a glass slide acting as the dielectric barrier and sample substrate. By applying an alternating voltage, a stable low-temperature plasma is formed between the discharge electrode and the glass surface28 and analytes (located on the glass slide) are desorbed and directly introduced into the MS. This initial configuration (pin-to-plate) was followed by LTP (pin-to-ring) and DBDI (ring-to-ring).Ambient MS applications of the ACaPI source include the analysis of the pesticide dichlorvos, with a handheld mass spectrometer.127 Pesticide testing using the ACaPI source involves so far, the use of hyphenated LC-MS or GC-MS techniques,126 or the use of solid-phase microextraction (SPME)128 with the SPME fibers used as substrates for subsequent thermal desorption and analyte ionization.
The first thorough study of LTP-MS applied to pesticide testing in fruit extracts deposited over a glass surface and fruit peels was performed by Wiley et al.132 Notably, the peak signal in LTP experiments was distinctly enhanced when the substrate was heated.118,132 LODs in the range from 0.2 to 200 ng g−1 were obtained for pesticides in spiked QuEChERS extracts of pepper, tomatoes and oranges using LTP-MS/MS with a heated substrate at ca. 100 °C.132 With a high-resolution Orbitrap MS instrument, LOQs in the range of 1–7 ng g−1 were obtained for a group of pesticides in grape and raspberry QuEChERS extracts, distinctly below the MRLs.133 Moreover, some authors have reported successful results in the direct analysis of samples without pretreatment. As an example, simple dilution applied to wines was enough to obtain LODs between 15 and 300 ng mL−1 for ten fungicides by LTP-MS/MS using an ion trap mass spectrometer.134 These values fulfilled the established MRL values, highlighting the usefulness of LTP-MS for the qualitative analysis of real samples with no sample treatment.
Wang et al.135 described thermal desorption LTP (TD-LTP) as a coupling between a thermal desorption sample injector and an LTP probe. A PTFE swab is used to wipe out a solid sample surface, or it is wetted by a liquid sample or extract, and finally the swab is inserted into the TD module. The desorbed molecules are transported by an air current into the LTP plasma jet, which interacts only with the sample in the gas phase, resulting in an increase in sensitivity and stability compared with conventional LTP. TD-LTP was used for the detection of 12 pesticides in broomcorn, using ultrasound-assisted extraction with methanol and the extract was deposited on a PTFE swab prior to TD-LTP-MS analysis. LODs ranged between 0.01 and 1 μg mL−1 for solvent standards.135
A different approach, proposed by Usmanov et al.,136 studied the desorption of low-volatility compounds by liquid–solid friction. Microdroplets (ca. 30 μm diameter) of water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were produced by a piezoelectric generator and spotted on the flat surface of an ultrasonically vibrating blade (Fig. 4g); microdroplet cavitation at the hitting interface was supposed to be the cause of the neutral desorption of analytes. The vaporized analytes were subsequently ionized by a modified LTP quartz capillary probe in which the pin electrode extends outside the capillary, so the plasma jet is cut off. The analytes gave strong signals, which were not observed when either the blade vibrator or the piezoelectric microdroplet generator was off. LODs ranged from 0.1 to 100 ng (Table 2).
1) were produced by a piezoelectric generator and spotted on the flat surface of an ultrasonically vibrating blade (Fig. 4g); microdroplet cavitation at the hitting interface was supposed to be the cause of the neutral desorption of analytes. The vaporized analytes were subsequently ionized by a modified LTP quartz capillary probe in which the pin electrode extends outside the capillary, so the plasma jet is cut off. The analytes gave strong signals, which were not observed when either the blade vibrator or the piezoelectric microdroplet generator was off. LODs ranged from 0.1 to 100 ng (Table 2).
One of the most attractive features of ambient ionization sources is the possibility to perform “in situ” analysis. The combination of LTP with a portable MS has been proven useful as a high throughput screening method to differentiate between organic and non-organic apples.60 Wiley et al.137 developed a handheld LTP source powered by a small battery and either helium or compressed air was used as the discharge gas. As expected, helium provided better LODs than air. Despite the reduction of gas and power consumption, the handheld source showed similar or slightly better analytical performance than the standard LTP, and LODs ranged between 0.001 and 0.9 ng, increasing up to 0.1–200 ng when a portable mini-MS was used.
3.4 Desorption atmospheric pressure photoionization (DAPPI)
DAPPI was developed by Haapala et al.138 for rapid surface analysis of compounds with a wide range of polarities (from polar to nonpolar analytes) (Fig. 5c).139 It involves the use of a heated nebulizer microchip, which supplies a heated jet of vaporized solvent, and a photoionization lamp. Sample spots on a surface are desorbed by the solvent jet, which is focused onto the surface, subsequently, analytes are ionized by APPI processes, and finally, they are detected by MS. Luosujärvi et al.140 studied species commonly found in environmental or food samples, including PAHs and pesticides (aldicarb, carbofuran, ditalimfos, imazalil, methiocarb, methomyl, oxamyl, pirimicarb, and thiabendazole). Three different spray solvents (with APPI dopants) were used in positive (acetone and toluene) and negative (anisole) ion modes. LODs for the studied pesticides ranged from 30 to 300 pg (corresponding to 0.14 to 1.4 pmol). Orange peel was directly analysed by cutting a small slice and attaching it onto the sample substrate; an abundant ion at m/z 297, corresponding to the protonated ion of imazalil, was observed and confirmed by MS/MS.Vaikkinen et al.141 compared the use of DAPPI and DESI to analyze neonicotinoid compounds (thiacloprid, acetamiprid, clothianidin, imidacloprid, and thiamethoxam). DAPPI gave signal-to-noise ratios from 2 to 11 times better than DESI. LODs ranged from 0.4 to 5.0 fmol for neat standard solutions. DAPPI was also used to detect thiacloprid on fresh rose leaves and turnip rape flowers. Analysis of plant material was performed by DAPPI with no further requirements of extraction or sample preparation.
4. Concluding remarks and future perspectives
The application of ambient desorption/ionization MS methods for the determination of different pesticides in foods has been extensively studied in recent years. One of the major attractive features of ambient MS sources is the possibility of direct analyte determination on sample surfaces (i.e. determination of contact pesticides on crops). The first consequence of real-time surface analysis of trace amounts of organic compounds is the ability to map chemicals on surfaces, and eventually, the acquisition of chemical images with moderate lateral resolution, which might be highly informative, for instance, to understand the application of agrochemicals on crops and their mechanisms (degradation, persistence, distribution, …). For instance, the use of DESI for MS imaging44 or the combination of laser ablation with FAPA-MS142 and LTP-MS143 may be cited as examples of this feature.In contrast, three main limitations may be observed for direct determination on foods with ambient MS methods. Firstly, direct surface analysis is affected by the nonhomogeneous pesticide distribution on the sample surface, which makes quantification efforts and method validation highly challenging. Secondly, in most ambient MS methods, only a small portion of the surface is investigated so the analysis may not achieve the required detection levels (MRL values, normally provided in mg kg−1 for the whole crop) depending on the studied surface (sweet spot effect). These limitations are usually avoided by the use of extraction techniques, such as surface liquid extraction, the use of dedicated procedures such as the QuEChERS procedure, or sampling the targeted surface with swabs, paper or foam disks wetted with an appropriate mixture of solvents, with the subsequent determination directly on the sampling substrate by an ambient MS method. A relatively low portion of the literature deals with quantitative analysis at low concentration levels, for instance with the use of ILIS. This issue yet remains one of the main challenges to solve given the lack of homogeneity in the distribution of pesticides in the sample. Thirdly, the occurrence of matrix effects in quantitative ambient MS methods should not be overlooked. There is a lack of thorough evaluation of matrix effects, although some studies have addressed this aspect.144
Finally, one of the most attractive features of ambient ionization sources is their use in portable mass spectrometers to perform in situ analysis. Amongst the ionization sources that have been coupled to a portable mass spectrometer we should mention DESI,42 PS,60 LTP137 and ACaPI.127 This is, definitely one of the most promising venues where ambient MS is expected to grow, as the availability of reliable portable MS instruments increases.8
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding from Consejería de Economía, Conocimiento, Empresas y Universidad, Regional Government of Andalucía, Spain (Project Ref. PY2018-1211) partially supported by EU FEDER funds. The authors also acknowledge funding by the Research Programme of the University of Jaen (Plan 2019–2020, Research programme “Acción 10”). This contribution has also received funding from the European Union Horizon 2020 research and innovation program under the Marie Sklodowska-Curie (MSCA) grant agreement number 840743.References
- USEPA, URL https://www.epa.gov/ingredients-used-pesticide-products/basic-information-about-pesticide-ingredients, last accessed: July 2020.
- EU, URL https://ec.europa.eu/food/plant/pesticides_en, last accessed: July 2020.
- EEA (European Environment Agency), Environmental indicator report, 2018, URL, https://www.eea.europa.eu/airs/2018/environment-and-health/pesticides-sales, last accessed: July 2020 Search PubMed.
- M. Farré and D. Barceló, Analysis of emerging contaminants in food, TrAC, Trends Anal. Chem., 2013, 43, 240–253 CrossRef.
- E. Pose-Juan, M. J. Sánchez-Martín, M. S. Andrades, M. S. Rodríguez-Cruz and E. Herrero-Hernández, Pesticide residues in vineyard soils from Spain: spatial and temporal distributions, Sci. Total Environ., 2015, 514, 351–358 CrossRef CAS.
- Chromatographic-Mass Spectrometric Food Analysis for Trace Determination of Pesticide Residues, ed. A. R. Fernández-Alba, Elsevier, 2004, Comprehensive Analytical Chemistry vol. 43 Search PubMed.
- J. J. Villaverde, B. Sevilla-Morán, C. López-Goti, J. L. Alonso-Prados and P. Sandín-España, Trends in analysis of pesticide residues to fulfil the European Regulation (EC) No. 1107/2009, TrAC, Trends Anal. Chem., 2016, 80, 568–580 CrossRef CAS.
- A. Molina-Díaz, M. Beneito-Cambra, D. Moreno-González and B. Gilbert-López, Ambient mass spectrometry from the point of view of Green Analytical Chemistry, Curr. Opin. Green Sustain. Chem., 2019, 19, 50–60 CrossRef.
- R. G. Cooks, Z. Ouyang, Z. Takats and J. M. Wiseman, Ambient mass spectrometry, Science, 2006, 311(5767), 1566–1570 CrossRef CAS.
- Z. Takáts, J. M. Wiseman, B. Gologan and R. G. Cooks, Mass spectrometry sampling under ambient conditions with desorption electrospray ionization, Science, 2004, 306(5695), 471–473 CrossRef.
- R. B. Cody, J. A. Laramée and H. D. Durst, Versatile new ion source for the analysis of materials in open air under ambient conditions, Anal. Chem., 2005, 77(8), 2297–2302 CrossRef CAS.
- R. Javanshad and A. R. Venter, Ambient ionization mass spectrometry: real-time, proximal sample processing and ionization, Anal. Methods, 2017, 9(34), 4896–4907 RSC.
- A. Venter, M. Nefliu and R. G. Cooks, Ambient desorption ionization mass spectrometry, TrAC, Trends Anal. Chem., 2008, 27(4), 284–290 CrossRef CAS.
- M. Z. Huang, C. H. Yuan, S. Y. Cheng, Y. T. Cho and J. Shiea, Ambient ionization mass spectrometry, Annu. Rev. Anal. Chem., 2010, 3, 43–65 CrossRef CAS.
- S. C. Cheng, C. Shiea, Y. L. Huang, C. H. Wang, Y. T. Cho and J. Shiea, Laser-based ambient mass spectrometry, Anal. Methods, 2017, 9(34), 4924–4935 RSC.
- P. Flanigan and R. Levis, Ambient Femtosecond Laser Vaporization and Nanosecond Laser Desorption Electrospray Ionization Mass Spectrometry, Annu. Rev. Anal. Chem., 2014, 7, 229–256 CrossRef CAS.
- M. E. Monge, G. A. Harris, P. Dwivedi and F. M. Fernández, Mass spectrometry: recent advances in direct open air surface sampling/ionization, Chem. Rev., 2013, 113(4), 2269–2308 CrossRef CAS.
- L. M. L. Nolet and B. K. Munjanja, Ambient Mass Spectroscopy Techniques in Food and the Environment, CRC Press, 2019 Search PubMed.
- M.-Z. Huang, C.-H. Yuan, S.-C. Chang, Y.-T. Cho and J. Shiea, Ambient ionization mass spectrometry, Annu. Rev. Anal. Chem., 2010, 3, 43–65 CrossRef CAS.
- H. Chen, G. Gámez and R. Zenobi, What Can We Learn from Ambient Ionization Techniques?, J. Am. Soc. Mass Spectrom., 2009, 20(11), 1947–1963 CrossRef CAS.
- D. J. Weston, Ambient ionization mass spectrometry: current understanding of mechanistic theory; analytical performance and application areas, Analyst, 2010, 135(4), 661–668 RSC.
- R. M. Alberici, R. C. Simas, G. B. Sanvido, W. Romão, P. M. Lalli, M. Benassi, I. B. S. Cunha and M. N. Eberlin, Ambient mass spectrometry: bringing MS into the “real world”, Anal. Bioanal. Chem., 2010, 398(1), 265–294 CrossRef CAS.
- M. Z. Huang, S. C. Cheng, Y. T. Cho and J. Shiea, Ambient ionization mass spectrometry: a tutorial, Anal. Chim. Acta, 2011, 702(1), 1–15 CrossRef CAS.
- G. A. Harris, A. S. Galhena and F. M. Fernández, Ambient sampling/ionization mass spectrometry: applications and current trends, Anal. Chem., 2011, 83(12), 4508–4538 CrossRef CAS.
- J. T. Shelley and G. M. Hieftje, Ambient mass spectrometry: approaching the chemical analysis of things as they are, J. Anal. At. Spectrom., 2011, 26(11), 2153–2159 RSC.
- Z. Takats, J. M. Wiseman and R. G. Cooks, Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology, J. Mass Spectrom., 2005, 40(10), 1261–1275 CrossRef CAS.
- D. R. Ifa, C. Wu, Z. Ouyang and R. G. Cooks, Desorption electrospray ionization and other ambient ionization methods: current progress and preview, Analyst, 2010, 135(4), 669–681 RSC.
- J. Chen, F. Tang, C. Guo, S. Zhang and X. Zhang, Plasma-based ambient mass spectrometry: a step forward to practical applications, Anal. Methods, 2017, 9(34), 4908–4923 RSC.
- A. Albert, J. T. Shelley and C. Engelhard, Plasma-based ambient desorption/ionization mass spectrometry: state-of-the-art in qualitative and quantitative analysis, Anal. Bioanal. Chem., 2014, 406(25), 6111–6127 CrossRef CAS.
- X. L. Ding and Y. X. Duan, Plasma-based ambient mass spectrometry techniques: the current status and future prospective, Mass Spectrom. Rev., 2015, 34(4), 449–473 CrossRef CAS.
- J. F. García-Reyes, B. Gilbert-López, A. Agüera, A. R. Fernández-Alba and A. Molina-Díaz, in Comprehensive Analytical Chemistry, ed. A. R. Fernández-Alba, Elsevier, 2012, ch. 8, vol. 58, pp. 339–366 Search PubMed.
- J. Hajslova, T. Cajka and L. Vaclavik, Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis, TrAC, Trends Anal. Chem., 2011, 30(2), 204–218 CrossRef CAS.
- DESI-MS, waters web page, URL https://www.waters.com/waters/en_US/DESI%3A-MS-Imaging-for-Biomedical-Research-/nav.htm?locale=en_US%26cid=134988839, last accessed, July 2020.
- M. W. F. Nielen, H. Hooijerink, P. Zomer and J. G. J. Mol, Desorption electrospray ionization mass spectrometry in the analysis of chemical food contaminants in food, TrAC, Trends Anal. Chem., 2011, 30(2), 165–180 CrossRef CAS.
- A. Gentili, S. Fanali and L. M. Rocca, Desorption electrospray ionization mass spectrometry for food analysis, TrAC, Trends Anal. Chem., 2019, 115, 162–173 CrossRef CAS.
- J. F. García-Reyes, A. U. Jackson, A. Molina-Díaz and R. G. Cooks, Desorption electrospray ionization mass spectrometry for trace analysis of agrochemicals in food, Anal. Chem., 2009, 81(2), 820–829 CrossRef.
- S. Gerbig, G. Stern, H. E. Brunn, R. A. Düring, B. Spengler and S. Schulz, Method development towards qualitative and semi-quantitative analysis of multiple pesticides from food surfaces and extracts by desorption electrospray ionization mass spectrometry as a preselective tool for food control, Anal. Bioanal. Chem., 2017, 409(8), 2107–2117 CrossRef CAS.
- C. Berchtold, V. Müller, L. Meier, S. Schmid and R. Zenobi, Direct detection of chlorpropham on potato skin using desorption electrospray ionization, J. Mass Spectrom., 2013, 48(5), 587–593 CrossRef CAS.
- L. M. Rocca, J. Cecca, N. L'Episcopo and G. Fabrizi, Ambient mass spectrometry: direct analysis of dimethoate, tebuconazole, and trifloxystrobin on olive and vine leaves by desorption electrospray ionization interface, J. Mass Spectrom., 2017, 52(11), 709–719 CrossRef.
- X. Z. Zhang, C. J. Li, S. S. Chen, X. J. Li, H. Han and X. D. Ma, Direct determination of atrazine residue on Chinese cabbage leaf using desorption electrospray ionization-tandem mass spectrometry and its application for diagnosing atrazine drift phytotoxicity, J. AOAC Int., 2009, 92(5), 1587–1592 CrossRef CAS.
- T. Cajka, K. Riddellova, P. Zomer, H. Mol and J. Hajslova, Direct analysis of dithiocarbamate fungicides in fruit by ambient mass spectrometry, Food Addit. Contam., 2011, 28(10), 1372–1382 CrossRef CAS.
- C. C. Mulligan, N. Talaty and R. G. Cooks, Desorption electrospray ionization with a portable mass spectrometer: in situ analysis of ambient surfaces, Chem. Commun., 2006, 1709–1711 RSC.
- L. Cardozo da Silva, I. Pereira, T. Colletes de Carvalho, J. F. Allochio Filho, W. Romão and B. Gontijo Vaz, Paper spray ionization and portable mass spectrometers: a review, Anal. Methods, 2019, 11, 999–1013 RSC.
- S. Gerbig, H. E. Brunn, B. Spengler and S. Schulz, Spatially resolved investigation of systemic and contact pesticides in plant material by desorption electrospray ionization mass spectrometry imaging (DESI-MSI), Anal. Bioanal. Chem., 2015, 407(24), 7379–7389 CrossRef CAS.
- H. Chen, A. Venter and R. G. Cooks, Extractive electrospray ionization for direct analysis of undiluted urine, milk and other complex mixtures without sample preparation, Chem. Commun., 2006, 2042–2044 RSC.
- Z. Zhou, M. Jin, J. Ding, Y. Zhou, J. Zheng and H. Chen, Rapid detection of atrazine and its metabolite in raw urine by extractive electrospray ionization mass spectrometry, Metabolomics, 2007, 3(2), 101–104 CrossRef CAS.
- S. Wang, F. Li, Y. Liu, H. Zhao and H. Chen, High-throughput screening of toxic substances by extractive electrospray ionization mass spectrometry and their identification via databank construction, Anal. Bioanal. Chem., 2019, 411, 4049–4054 CrossRef CAS.
- M. Li, B. Hu, J. Li, R. Chen, X. Zhang and H. Chen, Extractive electrospray ionization mass spectrometry toward in situ analysis without sample pretreatment, Anal. Chem., 2009, 81(18), 7724–7731 CrossRef CAS.
- H. Wang, J. Liu, R. G. Cooks and Z. Ouyang, Paper spray for direct analysis of complex mixtures using mass spectrometry, Angew. Chem., Int. Ed., 2010, 49(5), 877–880 CrossRef CAS.
- R. D. Espy, A. R. Muliadi, Z. Ouyang and R. G. Cooks, Spray mechanism in paper spray ionization, Int. J. Mass Spectrom., 2012, 325–327, 167–171 CrossRef CAS.
- E. M. McBride, P. M. Mach, E. S. Dhummakupt, S. Dowling, D. O. Carmany, P. S. Demond, G. Rizzo, N. E. Manicke and T. Glaros, Paper spray ionization: applications and perspectives, Trends Anal. Chem., 2019, 118, 722–730 CrossRef CAS.
- VeriSpray, PaperSpray ion source, Thermoscientific, URL https://assets.thermofisher.com/TFS-Assets/CMD/brochures/br-65405-verispray-paperspray-ion-source-br65405-en.pdf, accessed July 2020.
- S. L. Reeber, N. R. Wijeratne and M. L. Blackburn, Rapid measurement of agrochemicals by Paper Spray mass spectrometry (WP 303), poster of the 67th ASMS Conference on Mass Spectrometry and Allied Topics, Atlanta, Georgia, June 2–6, 2019 Search PubMed.
- S. Maher, F. P. M. Jjunju, D. E. Damon, H. Gorton, Y. S. Maher, S. U. Syed, R. M. A. Heeren, I. S. Young, S. Taylor and A. K. Badu-Tawiah, Direct analysis and quantification of metaldehyde in water using reactive paper spray mass spectrometry, Sci. Rep., 2016, 6, 35643 CrossRef CAS.
- S. L. Reeber, S. Gadi, S. B. Huang and G. L. Glish, Direct analysis of herbicides by paper spray ionization mass spectrometry, Anal. Methods, 2015, 7(23), 9808–9816 RSC.
- H. Evard, A. Kruve, R. Lõhmus and I. Leito, Paper spray ionization mass spectrometry: study of a method for fast-screening analysis of pesticides in fruits and vegetables, J. Food Compos. Anal., 2015, 41, 221–225 CrossRef CAS.
- T. Guo, W. Yong and Y. Dong, Automatically high-throughput quantification by paper spray ionization mass spectrometry for multiple pesticides in wine, Food Anal. Methods, 2019, 12(5), 1208–1217 CrossRef.
- A. C. Martins Moura, I. Neves Lago, C. Fernandes Cardoso, A. dos Reis Nascimento, I. Pereira and B. Gontijo Vaz, Rapid monitoring of pesticides in tomatoes (Solanum lycopersicum L.) during pre-harvest intervals by paper spray ionization mass spectrometry, Food Chem., 2020, 310, 125938 CrossRef.
- J. Liu, H. Wang, N. E. Manicke, J.-M. Lin, R. G. Cooks and Z. Ouyang, Development, characterization, and application of paper spray ionization, Anal. Chem., 2010, 82(6), 2463–2471 CrossRef CAS.
- S. Soparawalla, F. K. Tadjimukhamedov, J. S. Wiley, Z. Ouyang and R. G. Cooks, In situ analysis of agrochemical residues on fruit using ambient ionization on a handheld mass spectrometer, Analyst, 2011, 136(21), 4392–4396 RSC.
- Y. Ren, S. Chiang, W. Zhang, X. Wang, Z. Lin and Z. Ouyang, Paper-capillary spray for direct mass spectrometry analysis of biofluid samples, Anal. Bioanal. Chem., 2016, 408(5), 1385–1390 CrossRef CAS.
- F. Pu, W. Zhang, C. Han and Z. Ouyang, Fast quantitation of pyrazole fungicides in wine by ambient ionization mass spectrometry, Anal. Methods, 2017, 9(34), 5058–5064 RSC.
- I. Murray, G. Walker and M. S. Bereman, Improving the analytical performance and versatility of paper spray mass spectrometry via paper microfluidics, Analyst, 2016, 141, 4065–4073 RSC.
- T. C. Colletes, P. T. Garcia, R. B. Campanha, P. V. Abdelnur, W. Romão, W. K. T. Coltro and B. G. Vaz, A new insert sample approach to paper spray mass spectrometry: a paper substrate with paraffin barriers, Analyst, 2016, 141(5), 1707–1713 RSC.
- I. Pereira, S. R. M. Rodrigues, T. C. de Carvalho, V. V. Carvalho, G. S. Lobón, J. F. P. Bassane, E. Domingos, W. Romão, R. Augusti and B. G. Vaz, Rapid screening of agrochemicals by paper spray ionization and leaf spray mass spectrometry: which technique is more appropriate?, Anal. Methods, 2016, 8(31), 6023–6029 RSC.
- J. Liu, Y. He, S. Chen, M. Ma, S. Yao and B. Chen, New urea-modified paper substrate for enhanced analytical performance of negative ion mode paper spray mass spectrometry, Talanta, 2017, 166, 306–314 CrossRef CAS.
- Q. Wang, Y. Zheng, X. Zhang, X. Han, T. Wang and Z. Zhang, A silica coated paper substrate: development and its application in paper spray mass spectrometry for rapid analysis of pesticides in milk, Analyst, 2015, 140, 8048–8056 RSC.
- I. Pereira, M. Ferreira Rodrigues, A. Rodrigues Chaves and B. Gontijo Vaz, Molecularly imprinted polymer (MIP) membrane assisted direct spray ionization mass spectrometry for agrochemicals screening in foodstuffs, Talanta, 2018, 178, 507–514 CrossRef CAS.
- R. Narayanan, D. Sarkar, R. G. Cooks and T. Pradeep, Molecular Ionization from Carbon Nanotube Paper, Angew. Chem., Int. Ed., 2014, 53, 5936–5940 CrossRef CAS.
- J. Liu, H. Wang, R. G. Cooks and Z. Ouyang, Leaf spray: direct chemical analysis of plant material and living plants by mass spectrometry, Anal. Chem., 2011, 83(20), 7608–7613 CrossRef CAS.
- N. Malaj, Z. Ouyang, G. Sindona and R. G. Cooks, Analysis of pesticide residues by leaf spray mass spectrometry, Anal. Methods, 2012, 4(7), 1913–1919 RSC.
- B. Hu, P. K. So, H. Chen and Z. P. Yao, Electrospray ionization using wooden tips, Anal. Chem., 2011, 83(21), 8201–8207 CrossRef CAS.
- B. C. Yang, F. Wang, W. Deng, Y. Zou, F. Y. Liu, X. D. Wan, X. Yang, H. Liu and O. P. Huang, Wooden-tip electrospray ionization mass spectrometry for trace analysis of toxic and hazardous compounds in food samples, Anal. Methods, 2015, 7(14), 5886–5890 RSC.
- K. Hiraoka, K. Nishidate, K. Mori, D. Asakawa and S. Suzuki, Development of probe electrospray using a solid needle, Rapid Commun. Mass Spectrom., 2007, 21(18), 3139–3144 CrossRef CAS.
- Brochure of DPiMS-8060 ionization source, Shimadzu website, URL https://www.shimadzu.com/an/pdf/60_c146e369.pdf, last accessed, July 2020.
- K. Usui, E. Minami, Y. Fujita, E. Kubota, H. Kobayashi, T. Hanazawa, T. Yoshizawa, Y. Kamijo and M. Funayama, Application of probe electrospray ionization-tandem mass spectrometry to ultra-rapid determination of glufosinate and glyphosate in human serum, J. Pharm. Biomed. Anal., 2019, 174, 175–181 CrossRef CAS.
- K. Usui, E. Minami, Y. Fujita, H. Kobayashi, T. Hanazawa, Y. Kamijo and M. Funayama, A fast paraquat quantitation method in human serum using probe electrospray ionization–tandem mass spectrometry for emergency settings, J. Pharmacol. Toxicol. Methods, 2019, 100, 106610 CrossRef CAS.
- M. K. Mandal, T. Ozawa, S. Saha, M. M. Rahman, M. Iwasa, Y. Shida, H. Nonami and K. Hiraoka, Development of sheath-flow probe electrospray ionization mass spectrometry and its application to real time pesticide analysis, J. Agric. Food Chem., 2013, 61(33), 7889–7895 CrossRef CAS.
- M. Z. Huang, C. C. Zhou, D. L. Liu, S. S. Jhang, S. C. Cheng and J. Shiea, Rapid characterization of chemical compounds in liquid and solid states using thermal desorption electrospray ionization mass spectrometry, Anal. Chem., 2013, 85(19), 8956–8963 CrossRef CAS.
- C. Shiea, Y. L. Huang, D. L. Liu, C. C. Chou, J. H. Chou, P. Y. Chen, J. Shiea and M. Z. Huang, Rapid screening of residual pesticides on fruits and vegetables using thermal desorption electrospray ionization mass spectrometry, Rapid Commun. Mass Spectrom., 2015, 29(2), 163–170 CrossRef CAS.
- S.-C. Cheng, R.-H. Lee, J.-Y. Jeng, C.-W. Lee and J. Shiea, Fast screening of trace multiresidue pesticides on fruit and vegetable surfaces using ambient ionization tandem mass spectrometry, Anal. Chim. Acta, 2020, 1102, 63–71 CrossRef CAS.
- C.-W. Lee, H. Su, R.-H. Lee, Y.-P. Ling, Y.-D. Tsai, D.-C. Wu and J. Shiea, Point-of-care identification of organophosphates in gastric juice by ambient mass spectrometry in emergency settings, Clin. Chim. Acta, 2018, 485, 288–297 CrossRef CAS.
- C. W. Lee, H. Su, P. Y. Chen, S. J. Lin, J. Shiea, S. J Shin and B. H. Chen, Rapid identification of pesticides in human oral fluid for emergency management by thermal desorption electrospray ionization/mass spectrometry, J. Mass Spectrom., 2016, 51(2), 97–104 CrossRef CAS.
- J. H. Gross, Direct analysis in real time—a critical review on DART-MS, Anal. Bioanal. Chem., 2014, 406, 63–80 CrossRef CAS.
- C. Tendero, C. Tixier, P. Tristant, J. Desmaison and P. Leprince, Atmospheric pressure plasmas: a review, Spectrochim. Acta, Part B, 2006, 61, 2–30 CrossRef.
- J. J. Pérez, G. A. Harris, J. E. Chipuk, J. S. Brodbelt, M. D. Green, C. Y. Hampton and F. M. Fernández, Transmission-mode direct analysis in real time and desorption electrospray ionization mass spectrometry of insecticide-treated bednets for malaria control, Analyst, 2010, 135(4), 712–719 RSC.
- J. Schurek, L. Vaclavik, H. Hooijerink, O. Lacina, J. Poustka, M. Sharman, M. Caldow, M. W. F. Nielen and J. Hajslova, Control of strobilurin fungicides in wheat using direct analysis in real time accurate time-of-flight and desorption electrospray ionization linear ion trap mass spectrometry, Anal. Chem., 2008, 80(24), 9567–9575 CrossRef CAS.
- L. Zhang, W. Yong, J. Liu, S. Wang, Q. Chen, T. Guo, J. Zhang, T. Tan, H. Su and Y. Dong, Determination of dicyandiamide in powdered milk using direct analysis in real time quadrupole time-of-flight tandem mass spectrometry, J. Am. Soc. Mass Spectrom., 2015, 26(8), 1414–1422 CrossRef CAS.
- T. Guo, P. Fang, J. Jiang, F. Zhang, W. Yong, J. Liu and Y. Dong, Rapid screening and quantification of residual pesticides and illegal adulterants in red wine by direct analysis in real time mass spectrometry, J. Chromatogr. A, 2016, 1471, 27–33 CrossRef CAS.
- W. Yong, T. Guo, P. Fang, J. Liu, Y. Dong and F. Zhang, Direct determination of multi-pesticides in wine by ambient mass spectrometry, Int. J. Mass Spectrom., 2017, 417, 53–57 CrossRef CAS.
- F. J. Lara, D. Chan, M. Dickinson, A. S. Lloyd and S. J. Adams, Evaluation of direct analysis in real time for the determination of highly polar pesticides in lettuce and celery using modified Quick Polar Pesticides Extraction method, J. Chromatogr. A, 2017, 1496, 37–44 CrossRef CAS.
- M. Anastassiades, D. I. Kolberg, A. Benkenstein, E. Eichhorn, S. Zechmann, D. Mack, C. Wildgrube, I. Sigalov, D. Dörk and A. Barth, Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS/MS involving simultaneous extraction with methanol (QuPPe-method), http://www.crl-pesticides.eu/library/docs/srm/meth_QuPPe.pdf, accessed July 2020.
- S. E. Edison, L. A. Lin, B. M. Gamble, J. Wong and K. Zhang, Surface swabbing technique for the rapid screening for pesticides using ambient pressure desorption ionization with high-resolution mass spectrometry, Rapid Commun. Mass Spectrom., 2011, 25(1), 127–139 CrossRef CAS.
- S. E. Edison, L. A. Lin and L. Parrales, Practical considerations for the rapid screening for pesticides using ambient pressure desorption ionisation with high-resolution mass spectrometry, Food Addit. Contam., 2011, 28(10), 1393–1404 CrossRef CAS.
- S. E. Kern, L. A. Lin and F. L. Fricke, Accurate mass fragment library for rapid analysis of pesticides on produce using ambient pressure desorption ionization with high-resolution mass spectrometry, J. Am. Soc. Mass Spectrom., 2014, 25(8), 1482–1488 CrossRef CAS.
- E. Crawford and B. Musselman, Evaluating a direct swabbing method for screening pesticides on fruit and vegetable surfaces using direct analysis in real time (DART) coupled to an exactive benchtop orbitrap mass spectrometer, Anal. Bioanal. Chem., 2012, 403(10), 2807–2812 CrossRef CAS.
- X. Wang, X. Li, Z. Li, Y. Zhang, Y. Bai and H. Liu, Online coupling of in-tube solid-phase microextraction with direct analysis in real time mass spectrometry for rapid determination of triazine herbicides in water using carbon-nanotubes-incorporated polymer monolith, Anal. Chem., 2014, 86(10), 4739–4747 CrossRef CAS.
- G. A. Gómez-Ríos and J. Pawliszyn, Solid phase microextraction (SPME)-transmission mode (TM) pushes down detection limits in direct analysis in real time (DART), Chem. Commun., 2014, 50(85), 12937–12940 RSC.
- G. A. Gómez-Ríos, E. Gionfriddo, J. Poole and J. Pawliszyn, Ultrafast screening and quantitation of pesticides in food and environmental matrices by solid-phase microextraction-transmission mode (SPME-TM) and direct analysis in real time (DART), Anal. Chem., 2017, 89(13), 7240–7248 CrossRef.
- F. J. Andrade, J. T. Shelley, W. C. Wetzel, M. R. Webb, G. Gamez, S. J. Ray and G. M. Hieftje, Atmospheric pressure chemical ionization source. 1. Ionization of compounds in the gas phase, Anal. Chem., 2008, 80(8), 2646–2653 CrossRef CAS.
- F. J. Andrade, J. T. Shelley, W. C. Wetzel, M. R. Webb, G. Gamez, S. J. Ray and G. M. Hieftje, Atmospheric pressure chemical ionization source. 2. Desorption-ionization for the direct analysis of solid compounds, Anal. Chem., 2008, 80(8), 2654–2663 CrossRef CAS.
- M. C. Jecklin, G. Gamez, D. Touboul and R. Zenobi, Atmospheric pressure glow discharge desorption mass spectrometry for rapid screening of pesticides in food, Rapid Commun. Mass Spectrom., 2008, 22(18), 2791–2798 CrossRef CAS.
- J. T. Shelley, J. S. Wiley and G. M. Hieftje, Ultrasensitive ambient mass spectrometric analysis with a pin-to-capillary flowing atmospheric-pressure afterglow source, Anal. Chem., 2011, 83(14), 5741–5748 CrossRef CAS.
- X. Ding, X. Zhan, X. Yuan, Z. Zhao and Y. Duan, Microfabricated glow discharge plasma (MFGDP) for ambient desorption/ionization mass spectrometry, Anal. Chem., 2013, 85(19), 9013–9020 CrossRef CAS.
- B. Wang, X. Ding, Z. Zhao and Y. Duan, Method development for directly screening pesticide residues in foodstuffs using ambient microfabricated glow discharge plasma (MFGDP) desorption/ionization mass spectrometry, Int. J. Mass Spectrom., 2015, 377, 507–514 CrossRef CAS.
- H. W. Chen, J. H. Lai, Y. F. Zhou, Y. F. Huan, J. Q. Li, X. Zhang, Z. C. Wang and M. B. Luo, Instrumentation and characterization of surface desorption atmospheric pressure chemical ionization mass spectrometry, Chin. J. Anal. Chem., 2007, 35(8), 1233–1240 CAS.
- Z. Takats, I. Cotte-Rodriguez, N. Talaty, H. W. Chen and R. G. Cooks, Direct, trace level detection of explosives on ambient surfaces by desorption electrospray ionization mass spectrometry, Chem. Commun., 2005, 1950–1952 RSC.
- J. P. Williams, V. J. Patel, R. Holland and J. H. Scrivens, The use of recently described ionisation techniques for the rapid analysis of some common drugs and samples of biological origin, Rapid Commun. Mass Spectrom., 2006, 20(9), 1447–1456 CrossRef CAS.
- J. P. Williams and J. H. Scrivens, Rapid accurate mass desorption electrospray ionisation tandem mass spectrometry of pharmaceutical samples, Rapid Commun. Mass Spectrom., 2005, 19(24), 3643–3650 CrossRef CAS.
- H. Chen, J. Zheng, X. Zhang, M. Luo, Z. Wang and X. Qiao, Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination, J. Mass Spectrom., 2007, 42(8), 1045–1056 CrossRef CAS.
- J. Han, J. Q. Li, X. Zhang, B. Hu, M. B. Luo and H. W. Chen, Development of thermal dissociation atmospheric chemical ionization source for rapid mass spectrometry analysis of ambient samples, Chin. J. Anal. Chem., 2011, 39(2), 288–292 CAS.
- Y. Ouyang, X. Zhang, J. Han, X. Guo, Z. Zhu, H. Chen and L. Luo, Thermal dissociation atmospheric chemical ionization ion trap mass spectrometry with a miniature source for selective trace detection of dimethoate in fruit juices, Analyst, 2013, 138(2), 472–479 RSC.
- H. Wang, W. Sun, J. Zhang, X. Yang, T. Lin and L. Ding, Desorption corona beam ionization source for mass spectrometry, Analyst, 2010, 135(4), 688–695 RSC.
- X. Li, H. Wang, W. Sun and L. Ding, Desorption corona beam ionization coupled with a poly(dimethylsiloxane) substrate: Broadening the application of ambient ionization for water samples, Anal. Chem., 2010, 82(22), 9188–9193 CrossRef CAS.
- H. Wang, Y. Wu, B. Guo, W. Sun, L. Ding and B. Chen, Quantification of low-polar small molecules using room temperature ionic liquids matrix-assisted desorption corona beam ionization, Analyst, 2012, 137(17), 3982–3988 RSC.
- V. Horvatic, C. Vadla and J. Franzke, Discussion of fundamental processes in dielectric barrier discharges used for soft ionization, Spectrochim. Acta, Part B, 2014, 100, 52–61 CrossRef CAS.
- S. Brandt, F. D. Klute, A. Schütz and J. Franzke, Dielectric barrier discharges applied for soft ionization and their mechanism, Anal. Chim. Acta, 2017, 951, 16–31 CrossRef CAS.
- J. D. Harper, N. A. Charipar, C. C. Mulligan, X. Zhang, R. G. Cooks and Z. Ouyang, Low-temperature plasma probe for ambient desorption ionization, Anal. Chem., 2008, 80(23), 9097–9104 CrossRef CAS.
- A. Michels, S. Tombrink, W. Vautz, M. Miclea and J. Franzke, Spectroscopic characterization of a microplasma used as ionization source for ion mobility spectrometry, Spectrochim. Acta, Part B, 2007, 62, 1208–1215 CrossRef.
- H. Hayen, A. Michels and J. Franzke, Dielectric Barrier Discharge Ionization for Liquid Chromatography/Mass Spectrometry, Anal. Chem., 2009, 81, 10239–10245 CrossRef CAS.
- N. Na, M. Zhao, S. Zhang, C. Yang and X. Zhang, Development of a dielectric barrier discharge ion source for ambient mass spectrometry, J. Am. Soc. Mass Spectrom., 2007, 18(10), 1859–1862 CrossRef CAS.
- A. Michels, S. Tombrink, W. Vautz, M. Miclea and J. Franzke, Spectroscopic characterization of a microplasma used as ionization source for ion mobility spectrometry, Spectrochim. Acta, Part B, 2007, 62(11), 1208–1215 CrossRef.
- B. Gilbert-López, M. Schilling, N. Ahlmann, A. Michels, H. Hayen, A. Molina-Díaz, J. F. García-Reyes and J. Franzke, Ambient diode laser desorption dielectric barrier discharge ionization mass spectrometry of nonvolatile chemicals, Anal. Chem., 2013, 85(6), 3174–3182 CrossRef.
- M. M. Nudnova, L. Zhu and R. Zenobi, Active capillary plasma source for ambient mass spectrometry, Rapid Commun. Mass Spectrom., 2012, 26, 1447–1452 CrossRef CAS.
- Plasmion GmbH website, URL: https://www.plasmion.de, last accessed: July 2020.
- M. Mirabelli, J. C. Wolf and R. Zenobi, Pesticide analysis at ppt concentration levels: coupling nano-liquid chromatography with dielectric barrier discharge ionization-mass spectrometry, Anal. Bioanal. Chem., 2016, 408, 3425–3434 CrossRef CAS.
- J. C. Wolf, R. Etter, M. Schaer, P. Siegenthaler and R. Zenobi, Direct and Sensitive Detection of CWA Simulants by Active Capillary Plasma Ionization Coupled to a Handheld Ion Trap Mass Spectrometer, J. Am. Soc. Mass Spectrom., 2016, 27, 1197–1202 CrossRef CAS.
- M. F. Mirabelli, E. Gionfriddo, J. Pawliszyn and R. Zenobi, A quantitative approach for pesticide analysis in grape juice by direct interfacing of a matrix compatible SPME phase to dielectric barrier discharge ionization-mass spectrometry, Analyst, 2018, 143, 891–899 RSC.
- F. D. Klute, S. Brandt, P. Vogel, B. Biskup, C. Reininger, V. Horvatic, C. Vadla, P. B. Farnsworth and J. Franzke, Systematic Comparison between Half and Full Dielectric Barrier Discharges Based on the Low Temperature Plasma Probe (LTP) and Dielectric Barrier Discharge for Soft Ionization (DBDI) Configurations, Anal. Chem., 2017, 89, 9368–9374 CrossRef CAS.
- S. E. Spencer, B. G. Santiago and G. L. Glish, Miniature Flow-Through Low-Temperature Plasma Ionization Source for Ambient Ionization of Gases and Aerosols, Anal. Chem., 2015, 87, 11887–11892 CrossRef CAS.
- S. Martínez-Jarquín, A. Moreno-Pedraza, H. Guillén-Alonso and R. Winkler, Template for 3D Printing a Low-Temperature Plasma Probe, Anal. Chem., 2016, 88, 6976–6980 CrossRef.
- J. S. Wiley, J. F. García-Reyes, J. D. Harper, N. A. Charipar, Z. Ouyang and R. G. Cooks, Screening of agrochemicals in foodstuffs using low-temperature plasma (LTP) ambient ionization mass spectrometry, Analyst, 2010, 135(5), 971–979 RSC.
- A. Albert, A. Kramer, S. Scheeren and C. Engelhard, Rapid and quantitative analysis of pesticides in fruits by QuEChERS pretreatment and low temperature plasma desorption/ionization orbitrap mass spectrometry, Anal. Methods, 2014, 6(15), 5463–5471 RSC.
- M. Beneito-Cambra, P. Pérez-Ortega, A. Molina-Díaz and J. F. García-Reyes, Rapid determination of multiclass fungicides in wine by low-temperature plasma (LTP) ambient ionization mass spectrometry, Anal. Methods, 2015, 7(17), 7345–7351 RSC.
- S. Wang, Z. Wang, K. Y. Hou and H. Y. Li, Thermal desorption low temperature plasma ionization mass spectrometry for rapid and sensitive detection of pesticides in broomcorn, Chin. J. Anal. Chem., 2017, 45(2), 175–182 CAS.
- D. T. Usmanov, K. Hiraoka, H. Wada, S. Morita and H. Nonami, Desorption of low-volatility compounds induced by dynamic friction between microdroplets and an ultrasonically vibrating blade, Analyst, 2016, 141(4), 1398–1404 RSC.
- J. S. Wiley, J. T. Shelley and R. G. Cooks, Handheld low-temperature plasma probe for portable “Point-and-Shoot” ambient ionization mass spectrometry, Anal. Chem., 2013, 85(14), 6545–6552 CrossRef CAS.
- M. Haapala, J. Pól, V. Saarela, V. Arvola, T. Kotiaho, R. A. Ketola, S. Franssila, T. J. Kauppila and R. Kostiainen, Desorption atmospheric pressure photoionization, Anal. Chem., 2007, 79(20), 7867–7872 CrossRef CAS.
- T. J. Kauppila and R. Kostiainen, Ambient mass spectrometry in the analysis of compounds of low polarity, Anal. Methods, 2017, 9(34), 4936–4953 RSC.
- L. Luosujärvi, S. Kanerva, V. Saarela, S. Franssila, R. Kostiainen, T. Kotiaho and T. J. Kauppila, Environmental and food analysis by desorption atmospheric pressure photoionization-mass spectrometry, Rapid Commun. Mass Spectrom., 2010, 24(9), 1343–1350 CrossRef.
- A. Vaikkinen, H. S. Schmidt, I. Kiiski, S. Rämö, K. Hakala, M. Haapala, R. Kostiainen and T. J. Kauppila, Analysis of neonicotinoids from plant material by desorption atmospheric pressure photoionization-mass spectrometry, Rapid Commun. Mass Spectrom., 2015, 29(5), 424–430 CrossRef CAS.
- J. T. Shelley, S. J. Ray and G. M. Hieftje, Laser ablation coupled to a flowing atmospheric pressure afterglow for ambient mass spectral imaging, Anal. Chem., 2008, 80(21), 8308–8313 CrossRef CAS.
- A. Moreno-Pedraza, I. Rosas-Román, N. S. Garcia-Rojas, H. Guillén-Alonso, C. Ovando-Vázquez, D. Díaz-Ramírez, J. Cuevas-Contreras, F. Vergara, N. Marsch-Martínez, J. Molina-Torres and R. Winkler, Elucidating the distribution of plant metabolites from native tissues with laser desorption low-temperature plasma mass spectrometry imaging, Anal. Chem., 2019, 91(4), 2734–2743 CrossRef CAS.
- J. T. Shelley and G. M. Hieftje, Ionization matrix effects in plasma-based ambient mass spectrometry sources, J. Anal. At. Spectrom., 2010, 25, 345–350 RSC.
| This journal is © The Royal Society of Chemistry 2020 |
