Open Access Article
Open Access ArticleVersatile additively manufactured (3D printed) wall-jet flow cell for high performance liquid chromatography-amperometric analysis: application to the detection and quantification of new psychoactive substances (NBOMes)†
Hadil M.
Elbardisy
 ab,
Eduardo M.
Richter
ab,
Eduardo M.
Richter
 *ac,
Robert D.
Crapnell
*ac,
Robert D.
Crapnell
 a,
Michael P.
Down
a,
Michael P.
Down
 a,
Peter G.
Gough
a,
Tarek S.
Belal
a,
Peter G.
Gough
a,
Tarek S.
Belal
 d,
Wael
Talaat
b,
Hoda G.
Daabees
e and
Craig E.
Banks
d,
Wael
Talaat
b,
Hoda G.
Daabees
e and
Craig E.
Banks
 *a
*a
aFaculty of Science and Engineering, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, UK. E-mail: c.banks@mmu.ac.uk; Web: www.craigbanksresearch.com Tel: +44(0) 1612471196
bPharmaceutical Analysis Department, Faculty of Pharmacy, Damanhour University, Damanhour, 22511, Egypt
cInstitute of Chemistry, Federal University of Uberlandia, Av. João Naves de Avila, 2121, 38400-902, Uberlandia, MG, Brazil. E-mail: emrichter@ufu.br
dDepartment of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Alexandria University, Alexandria, 21521, Egypt
ePharmaceutical Chemistry Department, Faculty of Pharmacy, Damanhour University, Damanhour, 22511, Egypt
First published on 26th March 2020
Abstract
Additive manufacturing (AM/3D printing) is an emerging technology of vast applicability, receiving significant interest in a plethora of industrial domains and scientific research since it allows the rapid translation of designs produced via computer software, into AM/3D printed objects. To date, AM/3D printed devices have been examined for their utilisation as convenient and cost-effective tools towards the detection and quantification of prevalent drugs of abuse. Herein, a novel AM/3D printed wall-jet flow cell was fabricated specifically for employment in high performance liquid chromatography-amperometric detection (HPLC-AD) of various analytes (New Psychoactive Substances). Five sensing platforms were investigated, utilising different working electrodes, namely; screen-printed graphite electrodes (SPEs), AM/3D Proto-Pasta, AM/3D Black Magic, graphite sheet and AM/3D printed nanographite (NG)/polylactic acid (PLA) towards the detection of New Psychoactive Substances. The flow cell was also optimised with respect to the cell geometry demonstrating significant benefits such as simple production and operation and the ability to tailor the platform to a variety of working electrodes. The AM/3D printed sensing platforms were characterised towards the (electro) analytical detection of four N-benzylmethoxy-derivatives: 25F–NBOMe, 25C–NBOMe, 25B–NBOMe and 25I–NBOMe. Furthermore, the (electro) analytical performance of the flow cell was compared with the findings in our previous work comprising of a commercially available impinging jet flow cell. The SPEs and the graphite sheet were found to demonstrate superior electrochemical (analytical) sensitivity and higher reproducibility towards the quantification of the drugs in question, followed by the NG/PLA AM, Proto-Pasta and the Black Magic. The working electrodes that exhibited satisfactory (electro) analytical responses were employed for the analysis of NBOMe derivatives in three simulated blotter papers.
Introduction
Additive manufacturing (AM, 3D printing) is a rapidly growing technology that has had a major impact on manufacturing in the last few decades. It has evolved at an unprecedented rate, due to the freedom it offers in designing, rapid prototyping, mass customization, minimizing material waste and improving cost effectiveness.1 AM/3D printing builds three dimensional objects through digitally controlled deposition of materials in a layer-by-layer fashion until the final structure is created.2,3 To date, the most utilised AM/3D printing technique is fused deposition modelling (FDM). The simplicity and high speed of operation, as well as the availability of affordable 3D printers, has allowed FDM to become the most commonly used AM/3D printing technology.3 AM/3D printing can produce complex structures using a diverse range of materials (e.g., thermoplastics, metals, ceramics, living cells, etc.). This has enabled it to be utilized in a wide variety of fields, including aerospace,4 medicine,5 chemical engineering6 and environmental sciences.7 As this technology is continually advancing, it has opened new horizons in other research domains, such as analytical chemistry8,9 and electrochemistry.10,11 Examples of AM/3D printed electrochemical sensors and devices found in literature include; sensing electrodes,10 electrochemical cells,2 microfluidic devices,12 microneedles,13 supercapacitors14 and reaction ware for chemical synthesis and analysis.15The fabrication of flow cells for analytical purposes has been presented by commercial companies, such as Metrohm,16Antec Scientific,17 BASI®18 and ERC Co.19 There have also been reports in the scientific literature of fabricating flow cells for further applications in flow-injection analysis,20,21 electrochemiluminescent analysis,22 studying biological redox reactions,23 mechanistic and kinetic studies,24 electrochemical degradation of dye25 and environmental sciences.26 Interestingly, liquid chromatography with amperometric detection (LC-AD) has been one of the analytical approaches that has employed the use of flow cells; owing to its high sample throughput, affordable detector price and wide range of electroactive analytes that can be detected. Joynes et al. first reported a simple cell design incorporating a silicone-carbon membrane electrode for the quantitation of some organic and inorganic compounds.27 Thereafter, optimization of the geometrical configurations was reported, such as the classical thin-layer28 and the wall-jet flow cells.29 Further modification to these classical prototypes took place; such as wall-jet flow cells based on rotating disk electrodes.30,31 ESI Table S1† summarizes research articles described in literature dealing with flow cells manufactured for HPLC-AD. This includes the flow cells geometries and manufacturing materials, the utilised working electrodes, the analytes detected, and the pros and cons in the flow cells design.
Currently, there are several AM/3D printed electrochemical flow cells reported in the literature; for instance, chemiluminescence detection flow cells32 and flow cells for synthesis.33 To date, a fully AM/3D printed fabricated flow cell for LC-AD has not yet been reported. To this end, we report a novel multi-use wall-jet AM/3D printed flow cell that was constructed specifically for LC-AD. This flow cell is versatile and can incorporate electrodes of different materials. In this work, the tested working electrode substrates were screen-printed graphite macroelectrodes (SPEs), graphite sheets, and FDM AM/3D printable materials; including Proto-Pasta and Black Magic commercial conductive filaments and a homemade conductive filament synthesised from nanographite (NG) and polylactic acid (PLA) (NG/PLA). The proposed flow cell model and the sensitivity of the sensing platforms were evaluated by analyzing a mixture of four 2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine derivatives (NBOMe derivatives; 25F–NBOMe, 25C–NBOMe, 25B–NBOMe and 25F–NBOMe) as model analytes. NBOMEs are a new class of novel psychoactive substances, they are potent agonist of the 5-HT2A receptor and even doses in micrograms can produce psychoactive effects.34 These compounds display significant toxicological consequences as several deaths have been reported upon their intake.35,36 Accordingly, employing those NBOMes in this study can be beneficial for deduction of the analytical methodology of highest sensitivity for their quantification. Moreover, the analytical performance of the constructed AM/3D printed LC-AD flow cell was compared to the results obtained previously in our published report using a commercially available impinging jet flow cell for the electrochemical (amperometric) determination of NBOMe derivatives.37 Lastly, the working electrodes that demonstrated satisfactory electrochemical performance and sensitivity were utilised for the quantification of the four NBOMe derivatives in simulated blotters.
Experimental
Chemicals and materials
All chemicals were purchased at analytical grade from Sigma-Aldrich (UK) or Fluorochem Limited (UK) and used as received without any further purification. The investigated NBOMe derivatives (Scheme 1): 25F–NBOMe·HCl (2-(4-fluoro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine hydrochloride), 25C–NBOMe·HCl (2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine hydrochloride), 25B–NBOMe·HCl (2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine hydrochloride) and 25I–NBOMe·HCl (2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine hydrochloride) were synthesised, at Manchester Metropolitan University in accordance with Elbardisy et al.37 Lysergic acid diethylamide (LSD) (Scheme 1) was obtained from LGC GmbH (Germany) and was used as received without any further purification. All aqueous solutions were prepared with deionised water of resistivity ≥ 18.2 Ω cm. All solutions (unless stated otherwise) were vigorously degassed, for 10 minutes, with highly pure nitrogen to remove oxygen prior to analysis.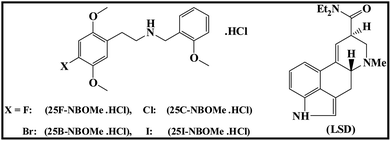 | ||
| Scheme 1 Chemical structures of 2,5-dimethoxy-N-(2-methoxybenzyl)phenethylamine (NBOMe) derivatives: 25F–NBOMe·HCl, 25C–NBOMe·HCl, 25B–NBOMe·HCl, 25I–NBOMe·HCl and lysergic acid diethylamide (LSD). The synthesis and characterisation of these compounds is reported in Elbardisy et al.37 | ||
Instrumentation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70% v/v] flowing at a rate of 2.5 mL min−1 (total run time: 12 min) with an injection volume of 10.0 μL. The HPLC was coupled to a home-made wall-jet AM/3D printed flow-cell housing the electrode to give the HPLC-AD system. The connection between the two parts was achieved via PTFE tubing (0.3 mm). Amperometric measurements were carried out using a Palmsens Emstat 3 (Palmsens BV, Netherlands) and controlled by PSTrace (version 4.4) software. All the amperometric measurements were carried out using the following parameters: potential (E, +1.0 V); equilibration time (tequilibration, 10.0 s); data interval (tinterval, 0.08 s); current range (100 nA to 1 mA) and total run time (trun, 5000 s).
70% v/v] flowing at a rate of 2.5 mL min−1 (total run time: 12 min) with an injection volume of 10.0 μL. The HPLC was coupled to a home-made wall-jet AM/3D printed flow-cell housing the electrode to give the HPLC-AD system. The connection between the two parts was achieved via PTFE tubing (0.3 mm). Amperometric measurements were carried out using a Palmsens Emstat 3 (Palmsens BV, Netherlands) and controlled by PSTrace (version 4.4) software. All the amperometric measurements were carried out using the following parameters: potential (E, +1.0 V); equilibration time (tequilibration, 10.0 s); data interval (tinterval, 0.08 s); current range (100 nA to 1 mA) and total run time (trun, 5000 s).
An ‘μAutolab type III’ (MetrohmAutolab, Netherlands) potentiostat/galvanostat interfaced to a PC loaded with NOVA 2.1 software was used to conduct chronoamperometry and cyclic voltammetric measurements for activation the AM/3D printed electrodes (Proto-Pasta and Black Magic). The measurements were performed using a 10 mL voltammetric cell and a conventional three electrode set-up.
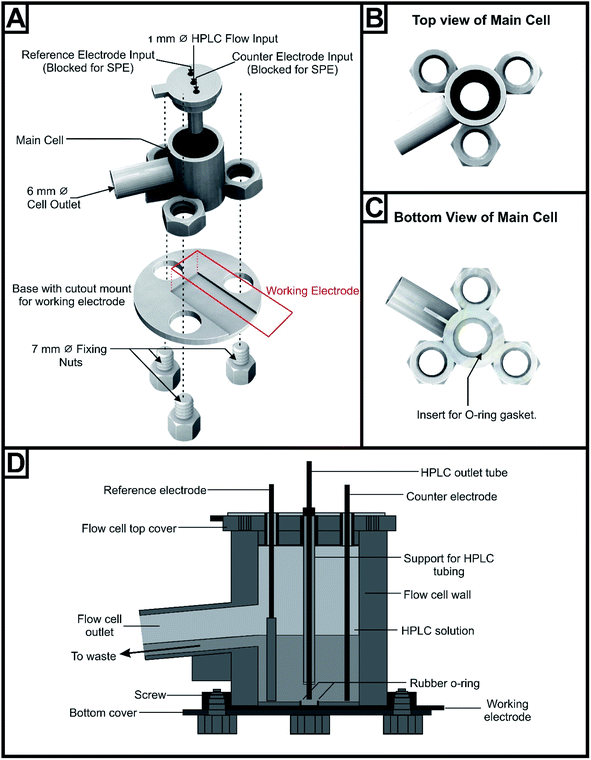
The AM/3D printed flow cell, with an internal volume of 1.2 mL, was composed of the following parts (see Fig. 1A). The top cover was comprised of three openings; two openings at the extremities for the counter and reference electrodes (these openings are left unoccupied in the case of using SPEs). The middle inlet was for the passage of the HPLC outlet PTFE tubing; this opening was extended downwards with a cylindrical support to hold the PTFE tubing close to the surface of the working electrode. The distance between the HPLC outlet PTFE tubing and the electrode surface was kept fixed at 1.5 mm. Access to the main body of the flow cell (height: 21.3 mm, internal diameter: 14 mm, external diameter: 17 mm) was provided by an orifice (fitted by a rubber O-ring) at the bottom for exposing the working electrode surface (shown in Fig. 1B and C). The electrode was situated at the bottom of the flow cell and exposed through the orifice, fitted with a rubber O-ring to prevent leakage. This orifice could have different internal diameters; 8.0 mm for the SPEs and 5.0 mm for the rest of the working electrodes. The flow cell had a cylindrical outlet (length: 16 mm) tilted at an angle of 5° that allowed the efflux of the mobile phase to pass easily out of the flow cell and decrease the internal pressure. It was connected to a waste container via rubber tubing. The outlet was helped by a support underneath to strengthen the outlet; making it more rigid and decreasing the possibility of its breakage. The bottom cover of the flow cell had a thin rectangular cavity, in which the working electrode was inserted, that accommodated a steel rectangular plate (11 × 34 mm2) for electrical contact of the working electrodes by their reverse side. This bottom cover had 3 holes for the insertion of the 3 screws to secure it tightly with the body of the flow cell. Fig. 1D represents a schematic cross-sectional diagram for the proposed AM/3D printed flow cell.
It should be noted that the reference and counter electrodes were utilised with all working substrates except the SPEs, which has the three electrode constituents (graphite working electrode, a graphite counter electrode and a Ag/AgCl reference electrode). The SPEs were connected to the potentiostat by the means of an edge connector, whilst connection to the rest of the working electrodes to the potentiostat was achieved by the means of crocodile clips in addition to the reference and counter electrodes.39 The electrical contact between the solution and the SPE surface was defined by the internal diameter of the bigger O-ring (8.0 mm). Likewise, the other working materials surfaces were limited by the smaller O-ring internal diameter (5.0 mm). However, the counter and reference electrodes had more contact with the solution as the mobile phase fills the half of the flow cell.
The SPEs utilised were fabricated in-house, as previously reported,41 and consisted of a 3.1 mm diameter graphite working electrode, a graphite counter electrode and a Ag/AgCl reference electrode. The SPE was cut wider than its width as shown in Fig. S1† in order to fill the rectangular cavity and decrease the chance of cell leakages; the dimension of the grey partition was measured at 34 × 11 mm2. It should be noted that a new SPE was used for each experiment performed. The commercial graphite sheet (18 × 11.5 cm2) was purchased from Panasonic Mouser Electronics (Mansfield, Texas). It was used as received from the company without any pre-treatments and cut to size, 34 × 11 mm2.
The commercial conductive filaments, Proto-Pasta (carbon black/PLA, filament diameter: 1.75 mm, print temperature 190–230 °C) and Black Magic (graphene/PLA, filament diameter: 1.75 mm, print temperature: 220 °C) were FDM 3D printed into a square using a conventional FDM ZMorphVX 3D printer (Z-Morph Industries, Poland). The 3D printed Proto-Pasta electrode was electrochemically activated as previously reported in the literature,42 by applying the potentials +1.4 V/200 s and −1.0 V/200 s in 0.5 mol L−1 NaOH using two 3D printed Proto-Pasta electrodes as the reference and counter electrodes. Similarly, the 3D printed Black Magic electrode was pre-treated following the methodology created by dos Santos et al.10 This was achieved by oxidation of the electrode at +1.8 V for 900 s and reduction from 0.0 V to −1.8 V at 50 mV s−1vs. saturated calomel electrode (SCE) in 0.1 mol L−1 PBS (pH 7.4).
NG/PLA AM-electrode was prepared using the following procedure. Nanographite (NG)/PLA filaments were fabricated by mixing NG and PLA, utilising a facile solution based mixing step. Briefly, the 3.75 g of mesoporous NG were dispersed within excess xylene (this mixture was sonicated for 10 minutes to ensure complete dispersion of the carbon in xylene) and heated under reflux at 150 °C for 3 hours. Following this, 11.25 g of PLA was added to the mixture and left for a further 3 hours. The resulting homogenous mixture was then precipitated in methanol, filtered under vacuum and left to dry overnight to ensure that any remaining solvent had evaporated. The resulting NG/PLA powder was then extruded with a Mini CTW twin-screw extruder (ThermoScientific) at a temperature of 200 °C and a screw speed of 30 rpm. The diameter of the filament (1.75 mm) was controlled with a specific die with a set diameter. The resulting filament was FDM 3D printed using the ZMorph VX® 3D printer with a direct drive extruder at a temperature of 190 °C.
The three AM/3D printed working electrodes were cut to the dimensions 34 × 11 × 1 mm3 and then their surfaces were entirely smoothed using wet sand paper. In order to achieve the three-electrode set-up for amperometric detection, all the used sensing platforms, except for the SPE, worked in conjunction with nickel wire which served as auxiliary/counter electrode and Proto-Pasta filament dipped into silver ink and left to dry overnight as the reference electrode.
Preparation of the mobile phase [acetonitrile: 5 mM ammonium formate–100 mM potassium chloride buffer (pH 7.0), 30
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70% v/v].
The buffer system (5 mM ammonium formate and 100 mM potassium chloride) was prepared and the pH was adjusted to pH 7.0 (±0.02) by dropwise addition of 0.2 M NaOH. Afterwards, appropriate proportions of the aqueous phase and the organic modifier were mixed to obtain a mobile phase of the desired ratio. Before running the HPLC, the mobile phase was vacuum filtered through a 0.45 mm pore filter paper.
70% v/v].
The buffer system (5 mM ammonium formate and 100 mM potassium chloride) was prepared and the pH was adjusted to pH 7.0 (±0.02) by dropwise addition of 0.2 M NaOH. Afterwards, appropriate proportions of the aqueous phase and the organic modifier were mixed to obtain a mobile phase of the desired ratio. Before running the HPLC, the mobile phase was vacuum filtered through a 0.45 mm pore filter paper.
Preparation of standard stock solution and calibration curve working solutions of NBOMEs for HPLC-AD. 10.0 mg of each NBOMe derivative were weighed accurately into one 20.0 mL clear glass volumetric flask and diluted to volume with mobile phase to give a stock solution containing 0.5 mg mL−1 of each drug. Working solutions for calibration standards were prepared by further dilution of the stock solution with the mobile phase to give solutions containing 10, 20, 50, 100, 150, 200 and 300 μg mL−1 of each analyte. Working solutions were injected directly into the HPLC and the current densities j (μA mm−2) of the studied drugs were plotted against their corresponding concentrations to construct the calibration curves.
Forensic application
Results and discussion
The overlay of cyclic voltammograms for a solution containing 100 μg mL−1 of each NBOMe derivative in 0.04 M Britton–Robinson buffer (pH 7.0) (depicted in Fig. S2†) illustrated that these compounds have two distinct oxidation peaks; peak I (E1 ≈ +0.815 V) and peak II (E2 ≈ +1.017 V), using screen printed graphite electrodes (SPEs) vs. Ag/AgCl reference electrode (scan rate: 50 mV s−1). The electrochemical oxidation mechanism of NBOMes was explained in detail by Andrade et al.43 Briefly, the first observed oxidation peak (peak I) develops as a result of the oxidation of the secondary amine into a primary amine. Whilst, the second oxidation peak (peak II) occurs due to the replacement of the halogen atom in the NBOMe compounds by a hydroxyl group, which afterwards produces a ketone.In our previous work,37 an HPLC-AD method employing a commercial impinging jet flow cell (3.3 × 6.0 × 3.3 cm, flow chamber volume = 8 μL, Metrohm UK, Runcorn, UK) was developed and applied for separation and quantification of the four phenethylamine derivatives (NBOMes), owing to the failure of the conventional voltammetric methods to separate them. In this study, we adopted the same HPLC-AD methodology for separation of the four drugs using our homemade AM/3D printed wall-jet flow cell and its performance characteristics were contrasted with those obtained from the commercial flow cell.37
AM/3D printed flow cell design optimization
Amperometric detection has been the most commonly investigated electrochemical detection technique in conjunction with liquid chromatography. It has been shown that the material of the working electrode and the geometry of the flow cell play a crucial role in the sensitivity and selectivity of the amperometric measurements.44 Two flow cell designs, the classical thin-layer and wall-jet, have dominated the literature in this area. The thin-layer cell geometry was characterised by the HPLC eluent flowing parallel to the active surface of the working electrode. However, in the wall-jet configuration the eluent flow-stream jets/impinges the surface of the working electrode perpendicularly, then it flows radially.44 The latter configuration has been proven to enhance the mass transport to the electrode surface, consequently improving the sensitivity.45,46 In addition, jetting of the solution on to the electrode surface minimised the electrode fouling and overcame memory effect by continuous cleaning of the working electrode surface.44,47 Because of these advantages, in this work an AM/3D printed wall-jet based flow cell was designed for use in conjunction with liquid chromatography. The key parameters involved in the design were the incorporation of different working electrode substrates, the sealing of the system and the distance between the solution inlet and working electrode surface. To achieve this, the working volume of the cell had to be increased to incorporate an external reference and counter electrode. This was due to the commercial flow cells being produced to only perform with the commercial SPEs that contained a working, reference and counter electrode.Firstly, the flow cell geometry was designed in a way to decrease the systems internal pressure. This was achieved by increasing the cylindrical outlet drainage diameter (6 mm) and tilting it at an angle of 5° from the horizontal. This enhanced the removal of the solution from the cell. The outlet was positioned 5 mm from the lower cell surface, which allowed only half of the cell capacity to be filled during the chromatographic run. This decreased the upward forces and pressure on the lower cell orifice, reducing the chance of leakages. A rubber O-ring was also used in the lower cell orifice to prevent leakages when the cell body was held together.
Secondly, the resolution of the printer, the AM/3D printing technique and the printing material were important during the development of the flow cell. To prevent leakages from the cell, a smooth uniform surface of the lower orifice was indispensable. A form 2 3D printer was operated by the SLA AM/3D printing technique alongside the photopolymer resin (Clear FLGPCL02) printing material. This offered AM/3D printed products of excellent quality and very smooth surfaces. The rough surfaces of the AM/3D printed electrodes utilised in this study, printed utilizing FDM technology (using commercially available and bespoke made filaments; Proto-Pasta, Black Magic and homemade NG/PLA AM-electrode) were thoroughly polished with wet sand paper before analytical measurements.
A critical factor of valuable consideration that was studied in most designed wall-jet flow cells is the distance between the HPLC outlet tubing and the electrode surface.48,49 The HPLC outlet PTFE tubing was fixed at variable distances above the electrode surface (0, 1, 2 and 3 mm) and the amperometric signal (current density j (μA mm−2)) of 500 μg mL−1 paracetamol was monitored utilising the flow cell with medium orifice. As seen in Fig. 2, the current density, j, was nearly unchanged over the range of the distances examined, emphasizing the robustness of the developed flow cell. Accordingly, the distance between the HPLC tubing outlet and the electrode surface was fixed at 1.5 mm throughout this study. Importantly, the durability and strength of the material of the flow cell was tested owing to the presence of organic solvent in the mobile phase that might cause weakness in the walls ending up with leakages. This was tested by leaving the assembled flow cell in contact with the mobile phase used in this study for three consecutive days and observing any changes occurs within the flow cell walls. No leaching of material or cracks within the body of the flow cell were noticed during this period.
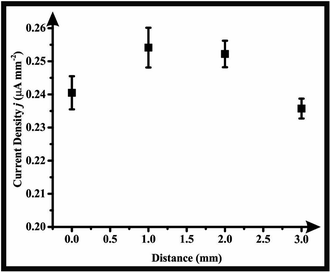 | ||
| Fig. 2 Studying the effect of the distance (mm) between the HPLC outlet PTEF tubing and the electrode surface on the amperometric response current density j (μA mm−2). | ||
The flow cell developed in this study had the advantage of being versatile, as its configuration permits the ease and rapid replacement of the three electrodes. This would allow the user to change to their preferred electrode without fabricating a new cell. Moreover, this simple design can be reproducibly fabricated. Also the flow cell is inexpensive to produce, has reduced manufacturing time, can tolerate high flow rates of the mobile phase without leakages (2.5 mL min−1) and can be tailored with different sizes to accommodate variety of electrodes.
Electroanalytical performance and validation parameters of the working electrodes used in this study
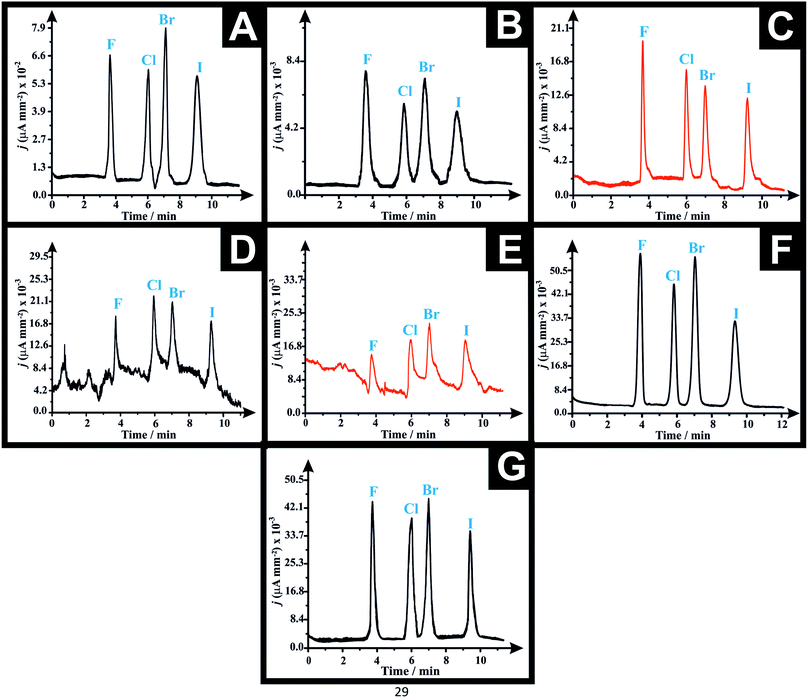
The HPLC-AD amperograms of a solution containing 300 μg mL−1 of the four NBOMe derivatives (i.e. 25F–NBOMe, 25C–NBOMe, 25B–NBOMe and 25I–NBOME) are depicted in Fig. 3. These were obtained utilising the developed AM/3D printed LC-AD wall-jet flow cell system in conjunction with the following working electrode substrates: SPEs (Fig. 3A), Proto-Pasta (CB/PLA) before activation (Fig. 3B), Proto-Pasta (CB/PLA) after activation (Fig. 3C), Black Magic (graphene/PLA) before pre-treatment (Fig. 3D), Black Magic (graphene/PLA) after pre-treatment (Fig. 3E), the graphite sheet (Fig. 3F) and NG/PLA AM-electrode (Fig. 3G). These working electrodes were chosen to exhibit the versatility of the AM/3D Printed flow cell, in addition to presenting the opportunity to realise a fully AM/3D printed sensor platform. The retention time of the eluted NBOMe derivatives in the depicted amperograms were as follows: 25F–NBOMe (tR = 3.84 min), 25C–NBOMe (tR = 5.97 min), 25B–NBOMe (tR = 7.02 min) and 25I–NBOMe (tR = 9.46 min) which were well corroborated with our previous work achieved using the commercial impinging jet flow cell.37The calibration curves for all the tested working substrates were constructed by plotting the current density j (μA mm−2) vs. the corresponding concentrations of each analyte (see Fig. S3A–G†). The validation parameters such as the intercepts (a), slopes (b), co-efficient of regression (r2) and limits of detection (LODs) for the analytical determination of the four NBOMe derivatives obtained for the different working electrodes using the proposed AM/3D printed flow cell HPLC-AD system are summarised in Table 1A–D. The LOD was calculated as per the following equation; LOD = 3.3Sa/b, where (Sa) is the standard deviation of the intercept and (b) is the slope of the regression line of the calibration curves of NBOMe derivatives. Repeatability tests (intra-day precision) were done by analyzing three different concentration levels of each derivative, three times (n = 3) on the same day, within the same laboratory, using the same instruments by the same analyst and the relative standard deviation percentages (RSD%) were calculated (Table S2†).
| Working electrode | Analyte | |||
|---|---|---|---|---|
| 25F–NBOMe | 25C–NBOMe | 25B–NBOMe | 25I–NBOMe | |
| a Intercept ± standard error. b Slope ± standard error. c n.d.: not detected. d LOD = (3.3 × Sa)/b; where Sa: standard deviation of intercept, b: slope of the regression line of the calibration curve. | ||||
| (A) Intercept of the regression line of the calibration curve (μA mm −2 ) | ||||
| SPE (AM/3D printed flow cell) linear range: 20–300 μg mL−1 | 5.17 ± 0.81 × 10−3 | 1.10 ± 0.82 × 10−3 | 1.88 ± 0.55 × 10−3 | −8.30 ± 7.48 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| SPE (commercial flow cell)37 linear range: 20–300 μg mL−1 | 1.93 ± 0.32 × 10−2 | 1.18 ± 0.15 × 10−2 | 1.13 ± 0.11 × 10−2 | 6.13 ± 0.80 × 10−3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (before activation) linear range: 50–300 μg mL−1 | 6.19 ± 13.4 × 10−5 | 3.83 ± 1.10 × 10−4 | 2.09 ± 1.20 × 10−4 | 1.33 ± 0.68 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (after activation) linear range: 20–300 μg mL−1 | 1.29 ± 0.07 × 10−3 | 6.68 ± 1.46 × 10−4 | 6.02 ± 0.67 × 10−4 | 4.47 ± 0.78 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (before pre-treatment) linear range: 50–300 μg mL−1 | 2.39 ± 1.38 × 10−3 | 1.72 ± 0.97 × 10−3 | 2.95 ± 1.38 × 10−3 | 2.83 ± 10.60 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (after pre-treatment) linear range: 50–300 μg mL−1 | 6.14 ± 7.13 × 10−3 | 4.13 ± 5.06 × 10−3 | 4.47 ± 4.14 × 10−3 | 2.84 ± 3.21 × 10−3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Graphite sheet linear range: 10–300 μg mL−1 | 2.51 ± 0.18 × 10−3 | 4.48 ± 2.12 × 10−4 | 1.09 ± 0.21 × 10−3 | 2.12 ± 1.35 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NG/PLA AM-electrode linear range: 20–300 μg mL−1 | −5.33 ± 6.79 × 10−4 | −1.21 ± 5.97 × 10−4 | −1.59 ± 0.56 × 10−3 | −9.38 ± 4.31 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (B) The slope of the regression line of the calibration curve (μA mL mm −2 μg−1) | ||||
| SPE (AM/3D printed flow cell) linear range: 20–300 μg mL−1 | 1.83 ±0.05 × 10−4 | 1.76 ± 0.05 × 10−4 | 1.79 ± 0.03 × 10−4 | 1.67 ± 0.05 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| SPE (commercial flow cell)37 linear range: 20–300 μg mL−1 | 7.49 ± 0.20 × 10−4 | 3.78 ± 0.09 × 10−4 | 4.88 ± 0.07 × 10−4 | 3.16 ± 0.05 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (before activation) linear range: 50–300 μg mL−1 | 2.40 ± 0.07 × 10−5 | 1.71 ± 0.06 × 10−5 | 2.23 ± 0.07 × 10−5 | 1.54 ± 0.04 × 10−5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (after activation) linear range: 20–300 μg mL−1 | 5.47 ± 0.04 × 10−5 | 4.39 ± 0.09 × 10−5 | 3.84 ± 0.04 × 10−5 | 3.61 ± 0.05 × 10−5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (before pre-treatment) linear range: 50–300 μg mL−1 | 3.53 ± 0.76 × 10−5 | 3.13 ± 0.53 × 10−5 | 2.24 ± 0.76 × 10−5 | 3.82 ± 0.58 × 10−5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (after pre-treatment) linear range: 50–300 μg mL−1 | 6.82 ± 3.92 × 10−5 | 4.52 ± 2.78 × 10−5 | 3.84 ± 2.28 × 10−5 | 4.25 ± 1.77 × 10−5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Graphite sheet linear range: 10–300 μg mL−1 | 1.89 ± 0.01 × 10−4 | 1.59 ± 0.01 × 10−4 | 1.38 ± 0.01 × 10−4 | 1.11 ± 0.01 × 10−4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NG/PLA AM-electrode linear range: 20–300 μg mL−1 | 1.48 ± 0.04 × 10−4 | 1.22 ± 0.04 × 10−4 | 1.29 ± 0.03 × 10−4 | 9.85 ± 0.26 × 10−5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (C) Coefficient of regression (r 2 ) | ||||
| SPE (AM/3D printed flow cell) linear range: 20–300 μg mL−1 | 0.997 | 0.997 | 0.999 | 0.997 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| SPE (commercial flow cell)37 linear range: 20–300 μg mL−1 | 0.997 | 0.997 | 0.999 | 0.999 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (before activation) linear range: 50–300 μg mL−1 | 0.997 | 0.996 | 0.997 | 0.998 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (after activation) linear range: 20–300 μg mL−1 | 0.999 | 0.998 | 0.999 | 0.999 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (before pre-treatment) linear range: 50–300 μg mL−1 | 0.878 | 0.920 | 0.745 | 0.935 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (after pre-treatment) linear range: 50–300 μg mL−1 | 0.501 | 0.467 | 0.486 | 0.658 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Graphite sheet linear range: 10–300 μg mL−1 | 0.999 | 0.999 | 0.999 | 0.999 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NG/PLA AM-electrode linear range: 20–300 μg mL−1 | 0.997 | 0.997 | 0.997 | 0.997 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (D) Limit of detection (LOD) (μg mL −1 ) | ||||
| SPE (AM/3D printed flow cell) linear range: 20–300 μg mL−1 | 14.67 ± 0.40 | 15.32 ± 0.44 | 10.20 ± 0.17 | 14.79 ± 0.44 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| SPE (commercial flow cell)37 linear range: 20–300 μg mL−1 | 14.22 ± 0.40 | 13.07 ± 0.31 | 7.63 ± 0.11 | 8.34 ± 0.13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (before activation) linear range: 50–300 μg mL−1 | 18.42 ± 0.54 | 21.19 ± 0.74 | 17.73 ± 0.56 | 14.58 ± 0.38 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Proto-Pasta (after activation) linear range: 20–300 μg mL−1 | 4.35 ± 0.03 | 10.96 ± 0.22 | 5.80 ± 0.06 | 7.17 ± 0.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (before pre-treatment) linear range: 50–300 μg mL−1 | n.d.c | n.d.c | n.d.c | n.d.c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Black Magic (after pre-treatment) linear range: 50–300 μg mL−1 | n.d.c | n.d.c | n.d.c | n.d.c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Graphite sheet linear range: 10–300 μg mL−1 | 3.16 ± 0.02 | 4.39 ± 0.03 | 5.02 ± 0.04 | 4.01 ± 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NG/PLA AM-electrode linear range: 20–300 μg mL−1 | 15.16 ± 0.41 | 16.11 ± 0.53 | 14.43 ± 0.34 | 14.44 ± 0.38 |
Forensic application
| Sample | Working electrode | Parameter | Drug recovered | |||
|---|---|---|---|---|---|---|
| LSD | 25F–NBOMe | 25B–NBOMe | 25I–NBOMe | |||
| a t R (min.) ± SD: retention time (minutes) for each eluted drug in the amperogram ± standard deviation (SD) of three determinations. b Mean % recovery ± SD of three determinations for each drug detected in each blotter paper. c % relative standard deviation of three determinations for each drug detected in each blotter paper. d % relative error; (+) = present in the blotter paper sample; (−) absent from the blotter paper sample. | ||||||
| (A) Blotter paper I | ||||||
| t R (min.) ± SDa | 2.78 ± 0.03 | 3.84 ± 0.05 | 7.02 ± 0.04 | 9.46 ± 0.06 | ||
| Blotter paper I | SPEs | Mean % R ± SDb | + | − | − | 100.1 ± 2.65 |
| RSD%c | + | − | − | 2.65 | ||
| E r (%)d | + | − | − | 0.1 | ||
| Activated Proto-Pasta | Mean % R ± SDb | + | − | − | 96.11 ± 3.34 | |
| RSD%c | + | − | − | 3.48 | ||
| E r (%)d | + | − | − | −3.89 | ||
| Graphite sheet | Mean % R ± SDb | + | − | − | 99.84 ± 1.42 | |
| RSD%c | + | − | − | 1.42 | ||
| E r (%)d | + | − | − | −0.16 | ||
| NG/PLA AM-electrode | Mean % R ± SDb | + | − | − | 96.90 ± 0.13 | |
| RSD%c | + | − | − | 0.13 | ||
| E r (%)d | + | − | − | −3.1 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| (B) Blotter paper II | ||||||
| t R (min.) ± SDa | 2.78 ± 0.05 | 3.84 ± 0.02 | 7.02 ± 0.09 | 9.46 ± 0.08 | ||
| Blotter paper II | SPEs | Mean % R ± SDb | + | 99.83 ± 2.12 | − | 103.0 ± 1.92 |
| RSD%c | + | 2.12 | − | 1.86 | ||
| E r (%)d | + | −0.17 | − | 3.00 | ||
| Activated Proto-Pasta | Mean % R ± SDb | + | 98.35 ± 1.51 | − | 98.74 ± 1.39 | |
| RSD%c | + | 1.54 | − | 1.41 | ||
| E r (%)d | + | −1.65 | − | −1.26 | ||
| Graphite sheet | Mean % R ± SDb | + | 100.3 ± 0.89 | − | 99.05 ± 1.71 | |
| RSD%c | + | 0.89 | − | 1.73 | ||
| E r (%)d | + | 0.27 | − | −0.95 | ||
| NG/PLA AM-electrode | Mean % R ± SDb | + | 102.8 ± 2.39 | − | 98.93 ± 0.53 | |
| RSD%c | + | 2.33 | − | 0.54 | ||
| E r (%)d | + | 2.76 | − | −1.07 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| (C) Blotter paper III | ||||||
| t R (min.) ± SDa | 2.78 ± 0.07 | 3.84 ± 0.04 | 7.02 ± 0.08 | 9.46 ± 0.08 | ||
| Blotter paper III | SPEs | Mean % R ± SDb | − | − | 98.47 ± 1.02 | − |
| RSD%c | − | − | 1.04 | − | ||
| E r (%)d | − | − | −1.53 | − | ||
| Activated Proto-Pasta | Mean % R ± SDb | − | − | 100.3 ± 3.33 | − | |
| RSD%c | − | − | 3.32 | − | ||
| E r (%)d | − | − | 0.26 | − | ||
| Graphite sheet | Mean % R ± SDb | − | − | 101.1 ± 1.43 | − | |
| RSD%c | − | − | 1.42 | − | ||
| E r (%)d | − | − | 1.05 | − | ||
| NG/PLA AM-electrode | Mean % R ± SDb | − | − | 100.4 ± 0.58 | − | |
| RSD%c | − | − | 0.58 | − | ||
| E r (%)d | − | − | 0.37 | − | ||
By careful investigation of the obtained results it was deduced that these four working substrates can work efficiently in combination with the proposed AM/3D printed flow cell for the electroanalytical quantification of a wide range of compounds in quality control labs. The best analytical performance was attributed to the SPEs and the graphite sheet. This is attributed to the increased electrochemically active sites presented by these two working electrode platforms, in addition to their high sensitivity, reproducibility, ease-of-use and scalability of production,55 noting that the former are mainly comprised of plastic with lesser electrochemically active sites.
Conclusions
Herein, we have reported for the first time the fabrication of a versatile wall-jet AM/3D printed flow cell that was designed especially for liquid chromatography-amperometric detection (LC-AD). The flow cell was optimised in terms of the design, geometry and the distance between the HPLC outlet PTFE tubing and the electrode surface. Additionally, the flow cell was evaluated by analyzing a mixture of four NBOMe derivatives utilising five working substrates; namely, SPEs, Proto-Pasta (before and after chemical activation), Black Magic (before and after chemical pre-treatment), graphite sheets and NG/PLA AM-electrode. The analytical performance of the AM/3D printed flow cell was compared with our previous report using a commercial impinging jet-flow cell incorporating SPEs as the working electrode material. Interestingly, the AM/3D printed flow cell had several advantages over the commercial systems. These included the simple geometrical configuration, short production time, low cost, higher sensitivity of the wall-jet design, efficient mass transport of the analyte onto the electrode surface, simple assembly, versatility toward working electrode substrates and high flow rate tolerance (2.5 mL min−1). This platform also provides flexibility in 3D designing, which permits simple modification of the flow cell configuration for the application to more specific electrode geometries and sizes.Regarding the working substrates tested in this study, it was demonstrated that the SPEs were the best working platform. They are not as sensitive as when using them incorporated into the commercial flow cell, however they still demonstrate a beneficial electrochemical response. The second best working platform was the graphite sheet; its sensitivity was comparable to the HPLC-DAD results published in our previous work, in addition to the sharp and symmetrical peaks of the obtained amperogram. The homemade conductive NG/PLA AM-electrode was the third-best sensor in term of sensitivity followed by the pre-treated Proto-Pasta electrode. The worst was the Black Magic working substrate that did not display a better amperometric response even after chemical pre-treatment. To sum up, the proposed AM/3D printed wall-jet flow cell accompanied with the SPEs, graphite sheet, NG/PLA AM-electrode and pre-treated Proto-Pasta working substrates can make a promising reliable and sensitive analytical tool that could be integrated within liquid chromatographic systems for the cost-effective electrochemical detection and quantification of various analytes in quality control and forensic laboratories.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We sincerely thank Newton-Mosharafa PhD Scholarship, funded by both the Egyptian Ministry of Higher Education and the British Council for supporting this research. Funding from the Engineering and Physical Science Research Council (Reference: EP/N001877/1) and a British Council Institutional Grant Link (No. 172726574) is acknowledged. Also, the authors would like to thank Dr Oliver B. Sutcliffe, Faculty of Science and Engineering, Manchester Metropolitan University, for providing the standard NBOMe drug samples to accomplish this work.References
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Q. Nguyen and D. Hui, Composites, Part B, 2018, 143, 172–196 CrossRef CAS.
- R. M. Cardoso, D. M. H. Mendonça, W. P. Silva, M. N. T. Silva, E. Nossol, R. A. B. da Silva, E. M. Richter and R. A. A. Muñoz, Anal. Chim. Acta, 2018, 1033, 49–57 CrossRef CAS PubMed.
- A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC.
- A. A. Sheikh, Virtual Phys. Prototyping, 2015, 10, 175–185 CrossRef.
- S. V. Murphy and A. Atala, Nat. Biotechnol., 2014, 32, 773 CrossRef CAS PubMed.
- C. Parra-Cabrera, C. Achille, S. Kuhn and R. Ameloot, Chem. Soc. Rev., 2018, 47, 209–230 RSC.
- C.-K. Su, P.-J. Peng and Y.-C. Sun, Anal. Chem., 2015, 87, 6945–6950 CrossRef CAS PubMed.
- B. Gross, S. Y. Lockwood and D. M. Spence, Anal. Chem., 2017, 89, 57–70 CrossRef CAS PubMed.
- C. L. Manzanares Palenzuela and M. Pumera, TrAC, Trends Anal. Chem., 2018, 103, 110–118 CrossRef CAS.
- P. L. dos Santos, V. Katic, H. C. Loureiro, M. F. dos Santos, D. P. dos Santos, A. L. B. Formiga and J. A. Bonacin, Sens. Actuators, B, 2019, 281, 837–848 CrossRef CAS.
- D. M. H. Mendonça, D. P. Rocha, G. S. V. Dutra, R. M. Cardoso, A. D. Batista, E. M. Richter and R. A. A. Munoz, Electroanalysis, 2019, 31, 771-777 Search PubMed.
- S. A. Gowers, V. F. Curto, C. A. Seneci, C. Wang, S. Anastasova, P. Vadgama, G. Z. Yang and M. G. Boutelle, Anal. Chem., 2015, 87, 7763–7770 CrossRef CAS PubMed.
- P. R. Miller, S. A. Skoog, T. L. Edwards, D. M. Lopez, D. R. Wheeler, D. C. Arango, X. Xiao, S. M. Brozik, J. Wang, R. Polsky and R. J. Narayan, Talanta, 2012, 88, 739–742 CrossRef CAS PubMed.
- M. Areir, Y. Xu, D. Harrison and J. Fyson, Mater. Sci. Eng., B, 2017, 226, 29–38 CrossRef CAS.
- M. D. Symes, P. J. Kitson, J. Yan, C. J. Richmond, G. J. T. Cooper, R. W. Bowman, T. Vilbrandt and L. Cronin, Nat. Chem., 2012, 4, 349–354 CrossRef CAS PubMed.
- https://www.metrohm.com/en-gb/products-overview/electrochemistry/cells-for-electrochemistry/, accessed August 2019.
- https://antecscientific.com/uhplc-with-electrochemical-detection-lc-ecd/flow-cells, accessed August 2019.
- https://www.basinc.com/products/ec/flowcells, accessed August 2019.
- http://www.erc-hplc.de/en/lc-detectors/ec-detector-ec-flowcells/electrochemical-flow-cells.html, accessed August 2019.
- J. S. Stefano, R. H. O. Montes, E. M. Richter and R. A. A. Muñoz, J. Braz. Chem. Soc., 2014, 25, 484–491 CAS.
- Y. Murakami, T. Takeuchi, K. Yokoyama, E. Tamiya, I. Karube and M. Suda, Anal. Chem., 1993, 65, 2731–2735 CrossRef CAS.
- Z. Jinjin, C. Ming, Y. Caixia and T. Yifeng, Analyst, 2011, 136, 4070–4074 RSC.
- H. Deng and G. J. V. Berkel, Electroanalysis, 1999, 11, 857–865 CrossRef CAS.
- Y. X. Chen, M. Heinen, Z. Jusys and R. J. Behm, Angew. Chem., Int. Ed., 2006, 45, 981–985 CrossRef CAS PubMed.
- L. Gomes, D. W. Miwa, G. R. P. Malpass and A. J. Motheo, J. Braz. Chem. Soc., 2011, 22, 1299–1306 CrossRef CAS.
- T. Noyhouzer and D. Mandler, Electroanalysis, 2013, 25, 109–115 CrossRef CAS.
- P. L. Joynes and R. J. Maggs, J. Chromatogr. Sci., 1970, 8, 427–433 CrossRef CAS.
- A. T. Hubbard and F. C. Anson, Electroanalytical Chemistry, ed. A. J. Bard, 1970, vol. 4, pp. 129–214 Search PubMed.
- J. Yamada and H. Matsuda, J. Electroanal. Chem. Interfacial Electrochem., 1973, 44, 189–198 CrossRef CAS.
- K. Brunt, C. H. P. Bruins, D. A. Doornbos and B. Oosterhuis, Anal. Chim. Acta, 1980, 114, 257–266 CrossRef CAS.
- B. Oosterhuis, K. Brunt, B. H. C. Westerink and D. A. Doornbos, Anal. Chem., 1980, 52, 203–205 CrossRef CAS.
- K. B. Spilstead, J. J. Learey, E. H. Doeven, G. J. Barbante, S. Mohr, N. W. Barnett, J. M. Terry, R. M. Hall and P. S. Francis, Talanta, 2014, 126, 110–115 CrossRef CAS PubMed.
- I. Lozano, C. López, N. Menendez, N. Casillas and P. Herrasti, J. Electrochem. Soc., 2018, 165, h688–h697 CrossRef CAS.
- P. Nikolaou, I. Papoutsis, M. Stefanidou, C. Spiliopoulou and S. Athanaselis, Drug Chem. Toxicol., 2015, 38, 113–119 CrossRef CAS PubMed.
- K. G. Shanks, T. Sozio and G. S. Behonick, J. Anal. Toxicol., 2015, 39, 602–606 CrossRef CAS PubMed.
- A. Al-Imam, Iran. J. Psychiatry Behav. Sci., 2018, 12, e9870 Search PubMed.
- H. M. Elbardisy, C. W. Foster, J. Marron, R. E. Mewis, O. B. Sutcliffe, T. S. Belal, W. Talaat, H. G. Daabees and C. E. Banks, ACS Omega, 2019, 4, 14439–14450 CrossRef CAS PubMed.
- Tinkercad|Create 3D digital designs with online CAD, 2019, https://www.tinkercad.com/, accessed February 2019.
- F. E. Galdino, C. W. Foster, J. A. Bonacin and C. E. Banks, Anal. Methods, 2015, 7, 1208–1214 RSC.
- C. W. Foster, H. M. Elbardisy, M. P. Down, E. M. Keefe, G. C. Smith and C. E. Banks, Chem. Eng. J., 2019, 122343, DOI:10.1016/j.cej.2019.122343.
- K. Y. Zuway, J. P. Smith, C. W. Foster, N. Kapur, C. E. Banks and O. B. Sutcliffe, Analyst, 2015, 140, 6283–6294 RSC.
- E. M. Richter, D. P. Rocha, R. M. Cardoso, E. M. Keefe, C. W. Foster, R. A. A. Munoz and C. E. Banks, Anal. Chem., 2019, 91, 12844–12851 CrossRef CAS PubMed.
- A. F. B. Andrade, S. K. Mamo and J. Gonzalez-Rodriguez, Anal. Chem., 2017, 89, 1445–1452 CrossRef CAS PubMed.
- M. Trojanowicz, Anal. Chim. Acta, 2011, 688, 8–35 CrossRef CAS PubMed.
- R. G. Compton, A. C. Fisher, M. H. Latham, C. M. A. Brett and A. M. C. F. O. Brett, J. Phys. Chem., 1992, 96, 8363–8367 CrossRef CAS.
- J. C. Ball, R. G. Compton and C. M. A. Brett, J. Phys. Chem. B, 1998, 102, 162–166 CrossRef CAS.
- K. Honeychurch, Separations, 2016, 3, 28 CrossRef.
- Z. Niegreisz, L. Szücs, J. Fekete, G. Horvai, K. Tóth and E. Pungor, J. Chromatogr. A, 1984, 316, 451–459 CrossRef CAS.
- H. Gunasingham and B. Fleet, Anal. Chem., 1983, 55, 1409–1414 CrossRef CAS.
- R. Guidelli, J. Electroanal. Chem. Interfacial Electrochem., 1971, 33, 291–302 CrossRef CAS.
- R. Guidelli, J. Electroanal. Chem. Interfacial Electrochem., 1971, 33, 303–317 CrossRef CAS.
- L. A. J. Silva, J. S. Stefano, R. M. Cardoso, N. S. Prado, P. H. T. Soares, E. Nossol, R. A. A. Munoz, L. Angnes and E. M. Richter, J. Electroanal. Chem., 2019, 833, 560–567 CrossRef CAS.
- J. L. Poklis, S. A. Raso, K. N. Alford, A. Poklis and M. R. Peace, J. Anal. Toxicol., 2015, 39, 617–623 CrossRef CAS PubMed.
- G. A. Souza, L. C. Arantes, T. J. Guedes, A. C. de Oliveira, P. A. Marinho, R. A. A. Muñoz and W. T. P. dos Santos, Electrochem. Commun., 2017, 82, 121–124 CrossRef CAS.
- A. García-Miranda Ferrari, C. W. Foster, P. J. Kelly, D. A. Brownson and C. E. Banks, Biosensors, 2018, 8, 53 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ay00500b |
| This journal is © The Royal Society of Chemistry 2020 |