Construction of a novel electrochemical biosensor based on a mesoporous silica/oriented graphene oxide planar electrode for detecting hydrogen peroxide†
Abstract
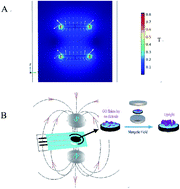
A constant magnetic field (CMF) was used to arrange the orientation of graphene oxide (GO) which was modified on a self-made screen-printed electrode. We evaluated the efficiency of this method for potential analytical application towards the sensing of hydrogen peroxide (H2O2). Mesoporous silica (MS)-encapsulated horseradish peroxidase (HRP) was immobilized on the electrode with vertically arranged GO to construct an H2O2 sensor (denoted as CMF/GO/HRP@MS). The linear range of the response of the CMF/GO/HRP@MS sensor to H2O2 was 0.1–235 μM, and the detection limit was as low as 0.01 μM. The results demonstrated that the vertical arrangement of GO resulting from the CMF on the electrode surface could increase the electron transfer rate. The excellent selectivity and anti-interference ability of this sensor to H2O2 in physiological samples may be attributed to the synergistic effect of mesoporous silica, GO and constant magnetic field.


 Please wait while we load your content...
Please wait while we load your content...