Open Access Article
Open Access ArticleDerivatization and rapid GC-MS screening of chlorides relevant to the Chemical Weapons Convention in organic liquid samples
Marja-Leena
Kuitunen
 *a,
Jorgelina Cecilia
Altamirano
*a,
Jorgelina Cecilia
Altamirano
 bc,
Peter
Siegenthaler
d,
Terhi Hannele
Taure
a,
Vesa Antero
Häkkinen
a and
Paula Sinikka
Vanninen
a
bc,
Peter
Siegenthaler
d,
Terhi Hannele
Taure
a,
Vesa Antero
Häkkinen
a and
Paula Sinikka
Vanninen
a
aVERIFIN, Department of Chemistry, University of Helsinki, P.O. Box 55, FIN-00014 Helsinki, Finland. E-mail: Marja-Leena.Kuitunen@alumni.helsinki.fi
bLaboratorio de Química Ambiental, Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales (IANIGLA, CCT-CONICET), Mendoza 5500, Argentina
cFacultad de Ciencias Exactas y Naturales, Universidad Nacional de Cuyo, Mendoza, Argentina
dFederal Office for Civil Protection FOCP, Spiez Laboratory, Analytical Chemistry Branch, CH-3700 Spiez, Switzerland
First published on 30th April 2020
Abstract
A simple derivatization technique was developed for the analysis of seven Schedule 3 chemicals and one Schedule 2 chemical listed in the Chemical Weapons Convention (CWC). Phosgene, phosphorus oxychloride, phosphorus trichloride, phosphorus pentachloride, thionyl chloride, sulfur monochloride and sulfur dichloride (Schedule 3) as well as arsenic trichloride (Schedule 2) were derivatized using 1-propanol in 40% pyridine solution for analysis with gas chromatography-mass spectrometry (GC-MS). Derivatization temperature and concentration of the derivatization solution were optimized for maximum derivatization recovery. The stabilities of the target analytes and their derivatives in different solvents were studied. The derivatization yield showed a linear response within the analyte concentration range of 0.1–2 mM (10–200 μg ml−1) with correlation coefficients >0.99 (r2), except for AsCl3 which did not show a linear response after derivatization. Good reproducibility with relative standard deviations (RSDs) from 3 to 13% was achieved. The derivatization recovery was 66% for phosgene and 67–80% for the P-containing chemicals phosphorus oxychloride, phosphorus trichloride and phosphorus pentachloride. Recommendations to use the method for screening the presence of these chemicals in organic liquid samples are given. The method is used when CWC-related samples are screened at VERIFIN.
Introduction
The Chemical Weapons Convention (CWC)1 entered into force in 1997, but chemical warfare agents (CWAs) are still topical. The threat to the use of these agents has regularly proved to be a source of news in this decade. The Organization for the Prohibition of Chemical Weapons (OPCW)2 implements the CWC for the prohibition of the development, production, stockpiling, and use of chemical weapons and on their destruction. During the last few years, the OPCW has needed to conduct inspections and samples were collected because of alleged uses of CWA.3–8 To this end, the OPCW has a network of designated laboratories and conducts proficiency tests (PTs) on a regular basis. These designated laboratories perform chemical verification analysis on the samples taken from the inspected sites and report if CWAs or CWC-related chemicals are identified.CWAs or toxic chemicals and their precursors are grouped into lists known as Schedule 1, 2, and 3 of the CWC, based on their risks to the convention and their degree of dual use, those in Schedule 1 having the greatest risk.9 Sample preparation procedures and analytical techniques used widely by the designated laboratories of the OPCW have been published as the Recommended Operating Procedures (ROPs), as monographs.10–13
The ROPs were not considered complete, and should never be, as new and better procedures will always emerge for sampling and analysis. Continuous efforts are required to keep these ROPs up-to-date. The increased pace of scientific and technological development further emphasizes this need. Additionally, there are gaps in the coverage of current procedures.14
Identification of chemicals by designated laboratories has to be unambiguous requiring separation and analysis by gas, liquid or ion chromatography coupled to spectrometric techniques or by nuclear magnetic resonance spectroscopy. However, some scheduled chemicals require derivatization due to their reactivity and/or inappropriate volatility for direct analysis with gas chromatographic techniques.
In Schedule 3, the three phosphorus containing inorganic chlorides phosphorus oxychloride (POCl3), phosphorus trichloride (PCl3), and phosphorus pentachloride (PCl5) are listed. Little has been published on the analysis of these chemicals: POCl3 and PCl3 were analyzed by GC-MS as well as by direct injection MS,15 as early as 1990. In addition, the Schedule 3 chemical sulfur dichloride (SCl2) was analyzed. In 2015 the derivatization of SCl2, as well as of sulfur monochloride (S2Cl2) using the electrophilic addition reaction with 3-hexyne was published.16 The reaction products were analyzed by GC-MS. For some reasons, the third sulfur chloride of the Schedule 3, thionyl chloride (SOCl2), was not analyzed.
Schedule 3 also contains the gaseous carbonyl dichloride (COCl2, phosgene), which is less corrosive than the mentioned phosphorus and sulfur containing chlorides. Phosgene can be analyzed directly in organic samples or extracts by GC-MS, taking into account that it elutes earlier than most of the organic solvents. Either headspace or split analysis should be used when this chemical is suspected in those types of samples.17 Phosgene can be formed by thermal decomposition of some plastics and chlorinated hydrocarbons. Phosgene also has many different industrial applications. For these reasons on-site methods using sensors and probes to detect phosgene in air have continuously been developed.18–24 Due to the instability of phosgene in air it has been collected in impingers and sorbent tubes containing a reagent to derivatize phosgene to a stable chemical.25 Chemical reactions of phosgene are similar to those of acid chlorides and it reacts easily with alcohols.26 Derivatization methods using mono- and bidentate nucleophiles have been reviewed.27,28 Phosgene was analyzed in air after derivatization with 2-aminothiophenol, 3,4-dimercaptotoluene (DMT), and 2-hydroxymethylpiperidine. The thermally stable derivatives were trapped with triethylamine onto Tenax® TA resin before thermal desorption (TD) GC-MS analysis.28 Later Juillet et al.29 validated sampling and analysis of phosgene using in situ derivatization with DMT on Tenax® GR tubes followed by analysis using TD–GC-MS with low limits of detection. This method is also presented in the latest ROP of air samples.30
Arsenic trichloride (AsCl3) is listed in Schedule 2. Due to its reactivity, it hydrolyzes rapidly in air, in water, or in water containing solvents. Wils15 analyzed directly the chemical with GC-MS without derivatization. Schoene et al.31 derivatized AsCl3 to thioarsenite with thioglycolic acid methyl ester prior to analysis with GC-MS and GC coupled with an atomic emission detector. In the ROP book 2011, the derivatization of AsCl3 in organic samples by trimethylsilylation was already discussed and derivatization with 1-butanethiol in presence of trimethylamine using an optimized method was proposed.12,13 Some years ago 1-buthyl-, 1-ethyl- and 1-propylthiol and some small dithiols were used for the derivatization of AsCl3 and lewisite 1 and 2. The chemicals and derivatization reagent were spiked in a water sample and the formed derivate analyzed using hollow fiber liquid phase micro extraction and GC-MS analysis.32
Thionyl chloride (SOCl2) reacts with alcohols to form chlorosulfite esters. The fate of these esters depends on the reaction conditions; especially on the stoichiometry, solvent, and amine base (e.g. pyridine is one of the most commonly used). If the appropriate ratios of alcohol and pyridine relative to SOCl2 are used, dialkyl sulfites are formed.33,34
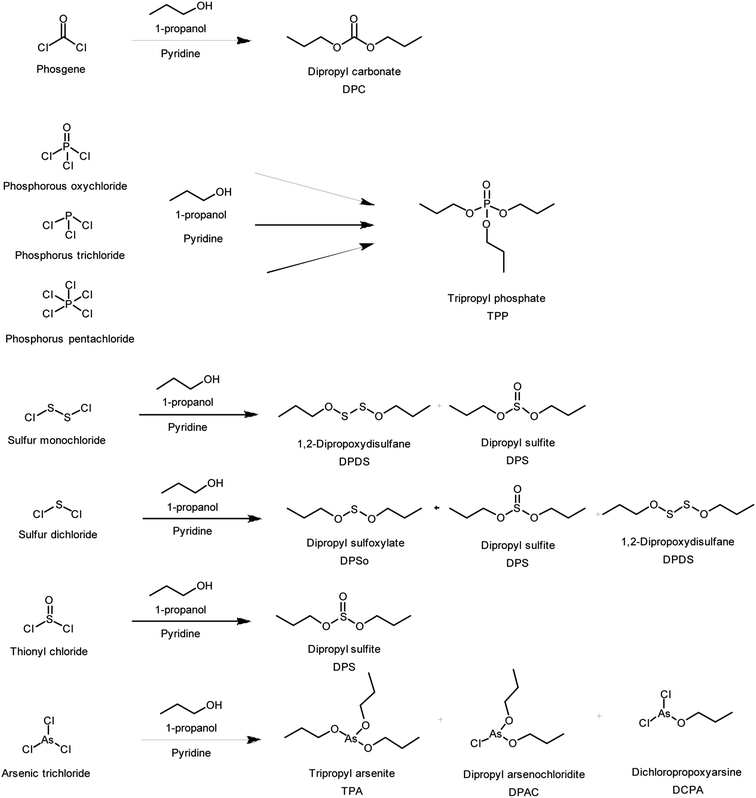
Niederhauser was the first to propose derivatization of scheduled chlorides with 1-propanol. The method was developed and tested in 2003 for COCl2, POCl3, PCl3, SOCl2, S2Cl2, and SCl2 in the Spiez Laboratory.35 In this study, the method was validated and further investigated in order to develop a more generic derivatization method by VERIFIN in collaboration with the Spiez Laboratory. POCl3, PCl3, PCl5, COCl2, SOCl2, S2Cl2, SCl2 and AsCl3 were derivatized with 1-propanol in pyridine for GC-MS analysis (Fig. 1 and Table 1). Derivatization conditions were optimized and the stabilities of the target analytes and the derivatives were investigated. Additionally, the derivatization recovery was determined for chlorides for which the commercially available derivatives dipropylcarbonate (DPC) and tripropylphosphate (TPP) were available as reference standards. Based on the optimization, a recommended sample preparation method was proposed for the derivatization of these chlorides in organic samples and extracts to identify them in OPCW proficiency tests and real samples.
| Analyte (schedule name, IUPAC name) | Chemical formula | CAS number | Schedule | Derivative | CAS number | MW |
|---|---|---|---|---|---|---|
| Phosgene, carbonyl trichloride | COCl2 | 75-44-5 | 3.A.01 | Dipropyl carbonate, DPC | 623-96-1 | 146 |
| Phosphorus oxychloride, phosphoryl trichloride | POCl3 | 10025-87-3 | 3.B.05 | Tripropyl phosphate, TPP | 513-08-6 | 224 |
| Phosphorus trichloride, trichlorophosphane | PCl3 | 7719-12-2 | 3.B.06 | Dipropyl sulfoxylate, DPSo | 3359-70-4 | 150 |
| Phosphorus pentachloride, pentachlorophosphane | PCl5 | 10026-13-8 | 3.B.07 | Dipropyl sulfite, DPS | 623-98-3 | 166 |
| Sulfur monochloride, hypochlorous dithioperoxyanhydride | S2Cl2 | 10025-67-9 | 3.B.12 | 1,2-Dipropoxydisulfane, DPDS | 3359-05-5 | 182 |
| Sulfur dichloride, hypochlorous thioanhydride | SCl2 | 10545-99-0 | 3.B.13 | Tripropyl arsenite, TPA | 15606-91-4 | 252 |
| Thionyl chloride, sulfurous dichloride | SOCl2 | 7719-09-7 | 3.B.14 | Dipropyl arsenochloridite, DPAC | 50880-08-5 | 229 |
| Arsenic trichloride, trichloroarsane | AsCl3 | 7784-34-1 | 2.B.07 | Dichloropropoxyarsine, DCPA | 3141-09-1 | 205 |
Experimental
Chemicals and reagents
POCl3 of 99% purity (Riedel-de-Haën, Seelze, Germany), PCl3, PCl5, and SOCl2 of 99% purity (Merck, Darmstadt, Germany), COCl2 and SCl2 of 99% purity (Fluka, Buchs, Switzerland), both S2Cl2 of 98% and AsCl3 of 99% of purity (Aldrich, St Louis, USA) were used as analytes. Tripropylphosphate (TPP) and dipropylcarbonate (DPC) were of 99% purity and purchased from Aldrich. 1 mM and 2 mM stock solutions of the analytes were prepared in n-hexane (99%, Merck). Toluene (99.5%, Merck), acetonitrile (99.5%, BDH, Poole, England) and dichloromethane (99.5%, Merck) were also tested as solvent for stock solutions. All solvents were dried with molecular sieve of 3 Å pore size (Technical, Ø 1.6 mm, BDH) before use to avoid hydrolysis of the target chemicals. A mixture of 1-propanol containing 10% and 40% (v/v) of pyridine was used as a derivatization reagent. 1-Propanol (>99%) and pyridine (99%) were purchased from Riedel-de-Haën. n-Octane (99%, Fluka) and n-dodecane (99%, ACROS Organics, Geel, Belgium) were used and tested as co-injection solvent to increase the solvent effect for better chromatographic resolution in GC-MS analyses. A solution of tributylphosphate (TBP) 1 mg ml−1 in dichloromethane was used as internal standard (IS) for quantitation and added to each sample before analysis. Analyte concentrations from 0.1 to 2.0 mmol l−1 were studied. Three parallel samples and method blank samples were prepared and analyzed.Derivatization procedure
Initially COCl2, POCl3, PCl3, SOCl2, S2Cl2, and SCl2 were diluted in dichloromethane and derivatized. 100 μl of 1-propanol solution containing 10% of pyridine was added to 100 μl of the analyte solution (100 or 10 μg ml−1). The vial was closed, shaked, and heated at 60 °C for 10 min before GC-MS analysis. For the same analytes as well as for PCl5 and AsCl3, derivatization of the analytes in n-hexane solution at the same conditions was also tested.Finally, 10 μl of the IS solution (TBP, 1 mg ml−1), 50 μl of n-octane, 50 μl of 1-propanol solution containing 40% pyridine and 50 μl of the analyte solution were added into a 300 μl glass vial insert in an autoinjector vial (Agilent Technologies, USA). The vial was immediately closed and vortexed for three seconds to ensure a proper mixing of the solution. The reaction was carried out at ambient temperature (22 °C) for 15 min before GC-MS analysis. Derivatization at −18 °C and 60 °C was also tested. For AsCl3, the best method was to increase the volume of 1-propanol solution to 100 μl and carry out the derivatization at 60 °C for 15 min.
Instrumentation and analysis
A HP MSD 5972 (Hewlett Packard, USA) mass selective detector coupled to a HP 5890 Series II GC (Hewlett Packard, USA) was used for analysis. The GC was equipped with an auto injector HP 6890 supplied by the same manufacturer. A DB-5 ms column (30 m length, 0.25 mm i.d., 0.25 μm film thickness; Agilent Technologies, USA) was used and the oven was programmed from the initial temperature 40 °C (1 min) to the final temperature of 280 °C at the rate of 10 °C min−1. The final temperature was kept for 15 min. Helium (Aga, Finland) was used as carrier gas with a flow rate of 1 ml min−1. The injector temperature was 250 °C and the injections of 1 μl were carried out in splitless mode with one minute splitless time. The MS was operated in electron ionization (EI) mode at 70 eV ionization energy. Samples were analyzed in full scan mode with a scan range of 40–550 m/z and scan speed of 1.5 scans per sec.The effects of the different experimental parameters on the derivatization yield were evaluated by comparing the peak areas of the derivatives with the peak area of the IS in the corresponding total ion chromatograms (TIC).
Results and discussion
Amount of derivatisation reagent and composition of the solution
In the first experiments 1-propanol containing 10% of pyridine was tested to derivatize COCl2, POCl3, PCl3, SOCl2, S2Cl2, and SCl2. Dichloromethane was used as the solvent for the analytes. Fig. 1 shows the reaction schemes and products identified and their names, CAS numbers and molecular weights are listed in Table 1. COCl2, SOCl2 and POCl3 reacted with 1-propanol forming the corresponding dipropyl or tripropyl derivatives DPC, DPS or TPP. PCl3 oxidized and formed TPP, the same derivate as for POCl3. Similarly, S2Cl2 and SCl2 derivatized to the corresponding dipropyl derivatives, DPDS and DPSo, and their oxidized form DPS, the same derivative as for SOCl2. In addition, DPDS was formed as a side product from SCl2.When the above-mentioned analytes as well as PCl5 and AsCl3 were dissolved in n-hexane instead of in dichloromethane, the derivatization was not successful for some of the analytes. Only COCl2, POCl3, and PCl3 showed the expected reactions reacted as listed in Table 1. For this reason, before next experiments all solvents and solvent mixtures were dried on a molecular sieve and stored in vials and sample bottles containing molecular sieve.
Derivatization experiments with POCl3 during the test phase had shown, that increasing the amount of pyridine, the reaction time as well as the reaction temperature did not have an effect on the yield of TPP.36 However, the volume of 1-propanol turned out to be critical. A large excess of 1-propanol containing 40% of pyridine was needed to derivatize 50 μl of 1 mM of the target chemical. This amount was also enough for the derivatization of COCl2, POCl3, PCl3, PCl5, SOCl2, SCl2 and S2Cl2, since a large excess of 1-propanol in pyridine compared to the amount of the target analyte was required. The same reaction products as shown in Fig. 1 were identified. PCl5 oxidized similarly as PCl3 forming TPP, the same derivate as for POCl3. When different concentrations of the analytes were tested, the responses were linear from 0.1 up to 2 mM indicating complete derivatization in the concentration range.
AsCl3 reacted with 1-propanol containing 40% of pyridine forming TPA but some amount of di- and mono propyl derivatives were also formed. The concentration of AsCl3 affected the ratio of the three derivatives TPA, DPAC, DCPA when 50 μl of 1-propanol containing 40% pyridine was used. At low AsCl3 concentrations (0.1–0.3 mM) TPA and DPAC were formed. When the concentration was 1 mM or higher, in addition to TPA and DPAC, DCPA was formed. The presence of DPAC and DCPA indicated that the derivatization was not complete. For this reason, the volume of 1-propanol solution was increased stepwise to 200 μl. The amounts of TPA and DPAC were increased in the reaction mixture but DCPA was still observed. DCPA was always formed when the AsCl3 concentration was 1 mM or higher. It could be shown that AsCl3 did not derivatize only to TPA. Finally, 100 μl of 1-propanol containing 40% pyridine for AsCl3 derivatization was a good compromise to produce TPA as the main reaction product without diluting the sample too much.
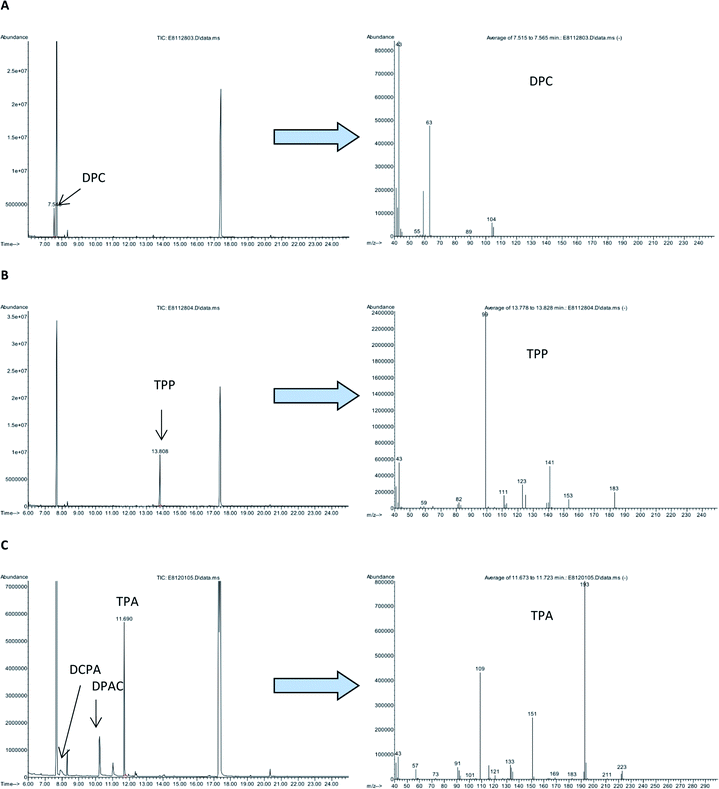
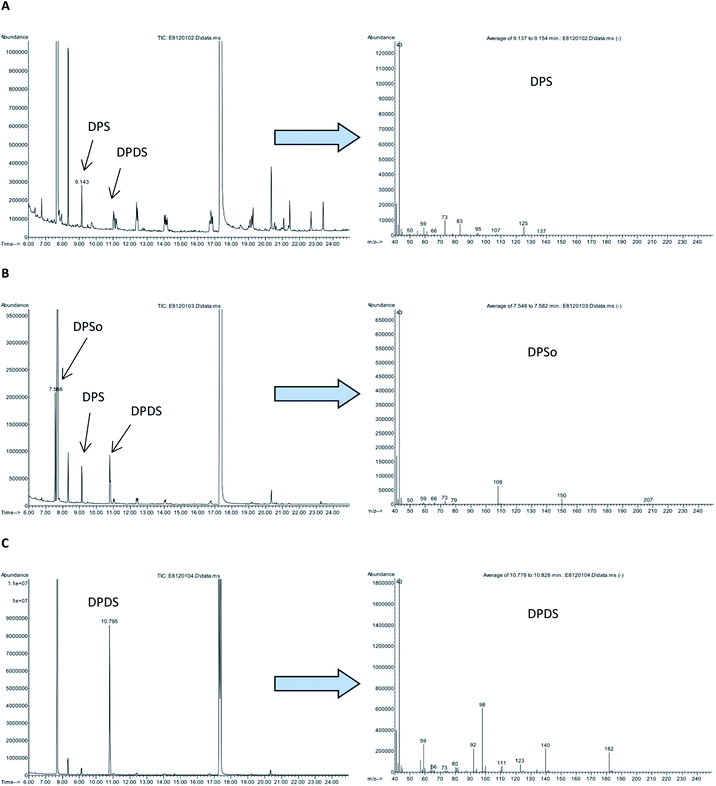
The total ion chromatograms (TICs) of the derivatized samples and EI mass spectra of the main derivatization products are shown in Fig. 2 and 3. Table 2 shows retention times of the main derivatives and the most intense ions of their mass spectra.
| Derivative | t R (min) | Mass spectrum |
|---|---|---|
| Dipropyl carbonate, DPC | 7.5 | 41 (25), 42 (15), 43 (100), 44 (4), 59 (23), 63 (56), 104 (7), 105 (5) |
| Tripropyl phosphate, TPP | 13.8 | 41 (11), 43 (23), 99 (100), 111 (6), 123 (12), 125 (7), 141 (21), 183 (8) |
| Dipropyl sulfoxylate, DPSo | 7.6 | 41 (25), 42 (2), 43 (100), 44 (3), 66 (1), 73 (2), 108 (10), 150 (3) |
| Dipropyl sulfite, DPS | 9.1 | 41 (16), 42 (5), 43 (100), 44 (4), 59 (4), 73 (8), 83 (6), 125 (4) |
| 1,2-Dipropoxydisulfane, DPDS | 10.8 | 41 (22), 43 (100), 57 (6), 59 (14), 92 (12), 98 (33), 140 (12), 182 (10) |
| Tripropyl arsenite, TPA | 11.7 | 41 (7), 43 (9), 109 (49), 116 (6), 133 (7), 151 (30), 193 (100), 194 (8) |
The intensities of the molecular ions (M+) and other specific ions were typically very low in the mass spectra of the examined sulfur-containing derivatives. In addition, the non-specific base ion m/z 43 is also prominently present in the mass spectra of hydrocarbons. Therefore, screening for low concentrations of the target derivatives in complex matrices containing hydrocarbon background using extracted ion chromatograms from low intensity ions will be problematic. The sensitivity and selectivity of the GC-MS analyses could be enhanced with GC-MS/MS experiments in selected reaction monitoring mode (SRM).
Derivatization temperature
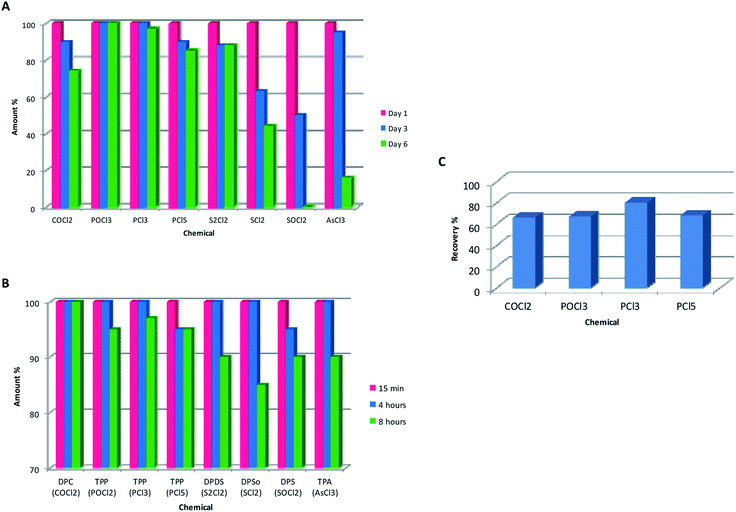
Besides derivatizing at ambient temperature, derivatization at −18 °C and 60 °C were also tested. Generally, there was no substantial difference between the derivatives and their amounts of different chlorides despite of the different reaction temperatures. The only exception was AsCl3: as the relative response of its derivative TPA increased notably when the derivatization was carried out at 60 °C.Peak splitting
Due to the sample composition (50% hexane, 20% pyridine, 30% 1-propanol) solvent effect (solvent focussing) did not work and peaks eluting after 10 min were splitting.37 At first split injection was tested to avoid peak splitting. Split ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, which should be suitable for the used column diameter,38 did not work. Splitting 1
20, which should be suitable for the used column diameter,38 did not work. Splitting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 lead to an acceptable peak shape but the sensitivity decreased clearly. Addition of a nonpolar solvent was tested. 50 μl of n-octane or n-dodecane was added to the derivatization mixture. With both hydrocarbons, the peak shapes of the derivatives were improved and the sensitivity was not significantly affected. The wide peak of C10 eluted at retention time 7.5–13.2 min interfering the detection of the derivatives, however.
3 lead to an acceptable peak shape but the sensitivity decreased clearly. Addition of a nonpolar solvent was tested. 50 μl of n-octane or n-dodecane was added to the derivatization mixture. With both hydrocarbons, the peak shapes of the derivatives were improved and the sensitivity was not significantly affected. The wide peak of C10 eluted at retention time 7.5–13.2 min interfering the detection of the derivatives, however.
Linearity, limit of detection, and precision
Linear regression was performed on the total peak area of ion chromatograms for each derivative. r2 values were >0.99 confirming linearity of the calibration range. In general, the calibration curves of the derivatives were linear in a concentration range from near the limit of detection (LOD) 0.1 mM to 2.0 mM except for the derivative TPA of AsCl3, where the linear range was limited. The LODs were calculated for a signal-to-noise ratio of 3 and were shown to be approx. 0.01 mM for all examined analytes. This corresponds to 1 μg ml−1 for COCl2.Precision was evaluated in terms of repeatability with the relative standard deviation percentage (RSD) of each derivate and TBP peak areas resulting from the analysis of 5 replicates. Repeatabilities were good: RSDs were lower than 7% for all chlorides except 13% for SCl2 (DPSo) and 10% for AsCl3 (TPA), respectively.
Stability of the chlorides
Due to the high reactivity of the tested chlorides, their stability in stock solutions was examined. Stock solutions of each chloride were prepared in dried n-hexane, toluene, acetonitrile and dichloromethane and stored in darkness at −18 °C. The solutions were derivatized and analyzed just after preparation as well as after three and six days. The changes in relative peak areas of the derivatives in GC-MS TIC after storage were compared to those obtained just after the preparation of the solution (relative response of the peak in the starting day = 100%).Fig. 4A shows that POCl3 was stable in n-hexane for six days, as the yield of its derivative was not decreased. All the other chlorides were less stable. The peak areas of PCl3 and PCl5 and COCl2 decreased 3%, 15%, and 26% in six days, which shows that PCl3 and PCl5 were also relatively stable in n-hexane. The peak area of the S2Cl2 derivative decreased 12% and SCl2 degraded significantly during storage resulting in a decrease of 56% of its derivative's peak area. Similarly, after storage of AsCl3 solution for six days and derivatization, only 16% of the derivative TPA could be found. However, the peaks of other derivatives of AsCl3 were significantly increased (DCPA 31%, DPAC 99%), suggesting that hydrolysis of AsCl3 was in progress. After six days of storage, the derivative of SOCl2 was no more detectable.
In general, similar results were obtained for these chlorides in acetonitrile, dichloromethane or toluene solutions. However, TPA was not formed when AsCl3 was dissolved in acetonitrile or dichloromethane solution. It seems obvious that one reason for this might be the higher solubility of water in acetonitrile or dichloromethane than in n-hexane.
Stability of the derivatives
The stability of the prepared derivatives using n-hexane, acetonitrile, dichloromethane, and toluene were monitored during eight hours. The derivatized samples were analyzed 15 min after preparation as well as after four and eight hours. The changes in relative peak areas were monitored as already described.Fig. 4B shows that the most stable derivative in n-hexane is DPC, the derivative of phosgene (COCl2). No degradation was observed after eight hours. TPP from PCl5 and DPS from SOCl2 were degraded roughly 5% after four hours and all the other derivatives showed a degradation rate of 3–15% within eight hours. Similar decomposition rates of the derivatives were observed in acetonitrile, dichloromethane and toluene. Nevertheless, it is recommended to analyze the derivatized samples just after derivatization.
As shown above, dryness of the solvents and reagents was important. It is therefore essential to close the vials immediately after addition of the chemicals with airtight caps particularly when sulfur-containing chlorides are derivatized. Moreover, to minimize hydrolysis of the analytes before derivatization, the sample should be added to the derivatization solution. For successful derivatization of samples, the following order of the derivatization steps is recommended: (1) n-octane (C8), (2) IS (if used), (3) 1-propanol containing 40% pyridine, (4) sample.
Derivatization recovery
DPC and TPP are commercially available and were used to determine the recoveries of the derivatization of COCl2, POCl3, PCl3, and PCl5. The chlorides were derivatized with 1-propanol containing 40% of pyridine and analyzed by GC-MS. The recovery was calculated by comparing the peak areas of the corresponding derivatives with those of standard solutions of DPC and TPP. The results are shown in Fig. 4C. The derivatization recovery of DPC was 66% for COCl2, while the TPP recoveries were 67% for POCl3, 80% for PCl3, and 68% for PCl5, respectively.Validation during testing
This method is in use at VERIFIN and has been tested successfully and reproducibly during several OPCW PTs. We have screened organic liquid samples with a solvent composition that may have contained the chlorides in question, e.g. dodecane sample in the 35th PT. When the method is validated during testing (on-the-job validation) the operation of the method is demonstrated by spiking the sample matrix with a known amount of chemicals (e.g. PCl3, SOCl2, and AsCl3). The sample preparation procedure and analysis has been carried out using the same approach as for the real PT sample usually at the same time. Identification of these spiked chlorides had ensured that the chemicals would have been identified in the PT sample, if it had been in the sample. For the present only AsCl3 has been used as a spiking chemical in the 25th PT, however.39,40Conclusions
A simple method was developed using 1-propanol with 40% of pyridine for the derivatization of CWC-related inorganic chlorides of Schedule 2 and 3. The derivatization temperature did not significantly affect derivatization of phosgene, POCl3, PCl3, PCl5, SOCl2, S2Cl2, and SCl2. However, the derivatization of AsCl3 was dependent on temperature leading to varying amounts of the different derivatives. When the temperature was increased, TPA yield was increased.In GC-MS chromatograms, the peaks of 1-propyl derivatives eluted separately from those of solvents and derivatization reagents. These derivatives shew unique mass spectra that can be used for their identification. A satisfactory chromatographic resolution was achieved by adding n-octane to increase the solvent effect in splitless injection mode. The linear dynamic range of the quantification was in the concentration range of 0.1–2.0 mM (10–200 μg ml−1). The Schedule 2 chemical AsCl3 did not show a linear response after derivatization. The derivatization recovery was 66% for phosgene and 67–80% for the P-containing chemicals POCl3, PCl3, and PCl5. The derivatives also seem to degrade over time and the extent of degradation depends on the analyte and the solvent. Therefore it is recommended to analyse the derivatized samples as soon as possible preferably within the same working day.
As shown, the described method suits well for screening of the presence of phosgene, phosphorus oxychloride, phosphorus trichloride, phosphorus pentachloride, thionyl chloride, sulfur monochloride, and sulfur dichloride as well as arsenic trichloride in organic solvent samples and solvent mixtures (e.g. organic solvent waste) providing there is no water present in the samples. If the derivative of interest is co-eluting with matrix components of the sample after GC-MS analysis, 1-propanol can be replaced by alternative alcohols for derivatization. This will result in different derivatives, shifted retention times (and different spectra) and the corresponding separation from the matrix components in GC analysis.
The best results are achieved when the chlorides were stored in dichloromethane or n-hexane. However, n-hexane is recommended as solvent, because the solubility of water in hexane is very low compared to the solubility of water in dichloromethane. The presence of water rapidly hydrolysed all target chemicals and the method could no longer be utilized. Therefore, it is recommended to dry the solvents using 3 Å molecular sieves before use. The stock solutions degrade over time and the degradation speed and amount depends on the analyte and the solvent. Therefore, dry fresh stock solutions should be used.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Organization for the Prohibition of Chemical Weapons, OPCW, the Swiss Federal Office for Civil Protection, and the Ministry for Foreign Affairs of Finland for financial support. Our former colleagues Mohamed Rafiq Bin Sulaiman and Martin Söderström are acknowledged for their input during the test phase of the method.References
- Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction, http://www.opcw.org/chemical-weapons-convention, accessed September 2019 Search PubMed.
- Organisation for the Prohibition of Chemical Weapons Headquarters, Johan de Wittlaan 32, 2517 JR, The Hague, The Netherlands, http://www.opcw.org, accessed Septem-ber 2019 Search PubMed.
- Final report of the United Nations mission to investigate allegations of the use of chemical weapons in the Syrian Arab Republic, Final report, December, 2013, https://unoda-web.s3.amazonaws.com/wp-content/uploads/2013/12/report.pdf, accessed July 2019 Search PubMed.
- Report of the OPCW fact-finding mission in Syria regarding alleged incident of 2 August 2016 as reported in the note verbale of the Syrian Arab Republic number 69 dated 16 August 2016, S/1444/2016, 21 Dec 2016 Search PubMed.
- Report of the OPCW fact-finding mission in Syria regarding alleged incident in Khan Shaykhun, the Syrian Arab Republic April 2017, S/1510/2017, 2 Jun 2017 Search PubMed.
- Report of the OPCW fact-finding mission in Syria regarding alleged incident in Ltamenah, the Syrian Arab Republic 30 March 2017, S/1548/2018, 2 Nov 2017 Search PubMed.
- Report of the OPCW fact-finding mission in Syria regarding alleged incident in Saraqib, the Syrian Arab Republic on 4 February 2018, S/1626/2018, 15 May 2018 Search PubMed.
- Report of the OPCW fact-finding mission in Syria regarding alleged incident in Ltamenah, the Syrian Arab Republic 24 and 25 March 2018, S/1636/2018, 13 June 2018 Search PubMed.
- R. Black, C. Timperley, H. Kiljunen, and M. Söderström, Chemicals, in Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, 2017 edn, 2017, pp. 5–32 Search PubMed.
- Recommended Operating Procedures for Sampling and Analysis in the Verification of Chemical Disarmament, ed. M. Rautio, Ministry for Foreign Affairs of Finland, Helsinki, 1993 Search PubMed.
- Recommended Operating Procedures for Sampling and Analysis in the Verification of Chemical Disarmament, ed. M. Rautio, Ministry for Foreign Affairs of Finland, Helsinki, 1994 Search PubMed.
- Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, ISBN 978-952-10-7407-3, 2011 edn, 2011 Search PubMed.
- Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, ISBN 978-951-51-3916-0, 2017 edn, 2017 Search PubMed.
- P. Vanninen and M. Söderström, Introduction, in Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, 2017 edn, 2017, pp. 1–3 Search PubMed.
- E. R. J. Wils, Fresenius. J. Anal. Chem., 1990, 338, 22–27 CrossRef CAS.
- D. R. Goud, D. Pardasani, A. K. Purohit, V. Tak and D. K. Dubey, Anal. Chem., 2015, 87, 6875–6880 CrossRef CAS PubMed.
- M. Söderström, O. Kostiainen, H. Koskela, A. Bossée, Y. Juillet, J. Xie, Q. Liu, M. Schär, P. Siegenthaler, U. Meier and J. Steen Jensen, Screening, in Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, 2017 edn, 2017, pp. 53–85 Search PubMed.
- A. Keil, H. Hernandez-Soto, R. J. Noll, M. Fico, L. Gao, Z. Ouyang and R. G. Cooks, Anal. Chem., 2008, 80, 734–741 CrossRef CAS PubMed.
- S. H. Lim, L. Feng, J. W. Kemling, C. J. Musto and K. S. Suslick, Nat. Chem., 2009, 1, 562–567 CrossRef CAS PubMed.
- G. J. Francis, D. B. Milligan and M. J. McEwan, Anal. Chem., 2009, 81, 8892–8899 CrossRef CAS PubMed.
- R. Niessner, Anal. Bioanal. Chem., 2010, 397, 2285–2288 CrossRef CAS PubMed.
- L. Feng, C. J. Musto, J. W. Kemling, S. H. Lim, W. Zhong and K. S. Suslick, Anal. Chem., 2010, 82, 9433–9440 CrossRef CAS PubMed.
- L. Chen, D. Wu, J. Kim and J. Yoon, Anal. Chem., 2017, 89, 12596–12601 CrossRef CAS PubMed.
- H. Xia, X. Xu and Q. Song, Anal. Chem., 2017, 89, 4192–4197 CrossRef CAS PubMed.
- K. Schoene, H. . J. Bruckert and J. Steinhanses, Fresenius' J. Anal. Chem., 1993, 345, 688–694 CrossRef CAS.
- O. Gyllenhaal and J. Vessman, J. Chromatogr., A, 1987, 395, 445–453 CrossRef CAS.
- R. M. Black and B. Muir, J. Chromatogr., A, 2003, 1000, 253–281 CrossRef CAS PubMed.
- B. Muir, D. B. Cooper, W. A. Carrick, C. M. Timperley, B. J. Slater and S. Quick, J. Chromatogr., A, 2005, 1098, 156–165 CrossRef CAS PubMed.
- Y. Juillet, C. Dubois, F. Bintein, J. Dissard and A. Bossée, Anal. Bioanal. Chem., 2014, 406, 5137–5145 CrossRef CAS PubMed.
- Y. Juillet, N. Lee Hoi Sim, C. Hoe Chee and M.-L. Kuitunen, Air samples, in Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, 2017 edn, 2017, pp. 207–221 Search PubMed.
- K. Schoene, J. Steinhanses, H. J. Bruckert and A. Koenig, J. Chromatogr., 1992, 605, 257–262 CrossRef CAS.
- M. Y. Cheh, H. C. Chua, F. B. Hopkins, J. R. Riches, C. M. Timperley and H. S. N. Lee, Anal. Bioanal. Chem., 2014, 406, 5103–5110 CrossRef CAS PubMed.
- http://reag.paperplane.io/00002684.htm, accessed Septem-ber 2019.
- C. M. Suter and H. L. Gerhart, Organic Syntheses, Coll., 1943, vol. 2, p. 112.A Search PubMed.
- A. Niederhauser, Screening auf niedrigmolekulare Verbindungen aus Liste 3 des Chemiewaffenüberein-kommens, Labornotiz NA 2003-01, Labor Spiez, Spiez, 2003 Search PubMed.
- M. R. Bin Sulaiman, Derivatisation and GC-MS analysis of selected Schedule 3 chemicals, OPCW Project No. L/ICA/116891/06, 2007 Search PubMed.
- K. Grob Jr, J. Chromatogr., 1981, 213, 3–14 CrossRef.
- D. Rood, J. Chromatogr. Sci., 1998, 36, 476–477 Search PubMed.
- K. Harju, P. Vanninen, E. Gómez Caballero, C. Hoe Chee and J. M. Moreno Sobrino, Basic requirements for method vali-dation, in Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, Helsinki, 2017 edn, 2017, pp. 31–51 Search PubMed.
- K. Norman, S. Johnson, H. Gregg, H. Kiljunen, C. Hoe Chee and P. Siegenthaler, A review of spiking chemicals used in the first 40 OPCW Proficiency Tests, in Recommended operating procedures for analysis in the verification of chemical disarmament, ed. P. Vanninen, University of Helsinki, P.O. Box 55, Helsinki, 2017 edn, 2017, pp. 753–807 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |