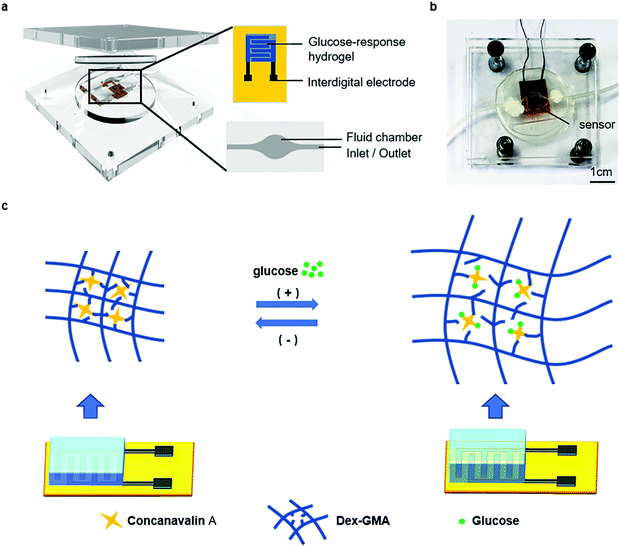
An enzyme-free capacitive glucose sensor based on dual-network glucose-responsive hydrogel and coplanar electrode†
Yingjie
Cai‡
a,
Dasheng
Yang‡
a,
Ruixue
Yin
 *a,
Yang
Gao
*a,
Yang
Gao
 *a,
Hongbo
Zhang
*a,
Hongbo
Zhang
 *a and
Wenjun
Zhang
b
*a and
Wenjun
Zhang
b
aSchool of Mechanical and Power Engineering, East China University of Science and Technology, Shanghai, China. E-mail: yinruixue@ecust.edu.cn; yanggao@ecust.edu.cn; hbzhang@ecust.edu.cn
bDivision of Biomedical Engineering, University of Saskatchewan, Saskatoon, Canada
First published on 7th October 2020
Abstract
Glucose sensors are vital devices for blood glucose detection in the diabetes care. Different from traditional electrochemical devices based on glucose oxidase, the glucose sensor based on the glucose-responsive hydrogel is more robust owing to its enzyme-free principle. However, integrating the high sensitivity, fast response, wide measuring range and low-cost fabrication into a hydrogel sensor is still challenging. In this study, we present a physical capacitive sensor, which consists of interdigital carbon electrodes (ICEs) fabricated by a direct laser writing technology and glucose-responsive hydrogel (DexG-Con A hydrogel) built by UV curing in situ. The dielectric property of DexG-Con A hydrogel changes accordingly with the change in environmental glucose concentration. Experimental results demonstrate that in a glucose concentration range of 0–30 mM, the proposed hydrogel sensor is capable of measuring the glucose level in a repeatable and reversible manner, showing a short responsive time of less than 2 min and a high sensitivity of 8.81 pF mM−1 at a glucose range of 0–6 mM. Owing to its simple fabrication process, low-cost and high performance, the proposed glucose sensor shows great potential on batch production for continuous glucose monitoring application.
Introduction
Diabetes mellitus is the third major chronic non-communicable disease that seriously threatens human health after tumors and vascular diseases. Diabetic patients are characterized by high blood glucose levels over a prolonged period. Blood glucose monitoring can reduce the risk of complications associated with long-term diabetes,1 and also contribute to better insulin therapy.2 Implantable glucose sensors with the function of continuous glucose monitoring (CGM) have attracted great attention. They provide important information for diabetic control, including the instantaneous real-time display of the glucose level and glucose change rate, alerts and alarms for actual or impending hypo- and hyperglycemia, “24/7” coverage, and the ability to characterize glycemic variability.3 Artificial pancreatic systems that aim for insulin-secreting-cell-like insulin delivery, having been in development for several years, also require a high-performance glucose sensor to integrate into close-loop circuits.The traditional glucose sensor relied on the conversion of glucose into gluconolactone and hydrogenperoxide (H2O2) by using glucose oxidase (GOx), which may generate interference and finally cause false read-outs of CGM devices.4 In addition, the enzyme sensors are consumptive and irreversible, which easily causes large drifts when the external demand changes. In order to overcome the instability and low accuracy of enzyme-based sensors, lot of substrates (such as carbon nanotubes, inorganic nanostructures and robust hydrogels) have been proposed for GOx immobilization.5,6 However, these methods may increase the complexity in sensor fabrication, require high operation skills,7 and involve high costs. Therefore, non-enzymatic glucose sensors have been developed alternatively. Metal catalysts (such as Ag, Pt and Au, or some metallic oxides like CuO, NiO, MnO and ZnO) were used to directly catalyze glucose.8–10 Unfortunately, these sensors usually cannot avoid the disturbance of active molecules like dopamine, uric acid, ascorbic acid, KCl, NaCl and fructose. They also have a small glucose detecting range,11,12 which cannot cover a target range of 4–10 mM for the blood glucose control of diabetic patients.13
Glucose-responsive hydrogels based on reversible specific binding between glucose-binding molecules and glucose are good candidates as an enzyme-free detection element for the glucose sensor, benefitting from its biocompatibility, rapid responsibility and easily adjusted network structure.14 The swelling of such a responsive hydrogel can be programmed according to an external glucose stimulus, leading to the corresponding change of physical property (e.g., refractive index, vibration, elastic modulus, resistance), which can be measured as a sensing signal. Phenylboronic acid (PBA)15–18 and concanavalin A (Con A)-based glucose-responsive hydrogels19–21 are two widely investigated groups to fabricate hydrogel-based enzyme-free glucose sensors. PBA is a chemically synthesized agent that shows reversible affinity to compounds with adjacent hydroxyl groups. Con A is a glucose-binding lectin extracted from the Jack bean, which exhibits strong reversible affinity for the pyranose ring with unmodified –OH at the C-3, C-4 and C-6 positions.22 Compared with the PBA-contained hydrogel, the Con A-based hydrogel has better biocompatibility and a much stronger specificity for sugar.23 Until now, Con A-based hydrogels in the form of a hollow fiber and thin film have been developed as optical glucose sensors.24,25 Nevertheless, integrating high sensitivity, wide measuring range and low-cost fabrication into the Con A-based hydrogel sensor is still challenging.
In this study, for the first time, we present a physical capacitive sensor integrated by glycidyl methacrylate dextran (DexG)-Con A hydrogel and interdigital carbon electrodes (ICEs) to address the aforementioned issues. The DexG-Con A hydrogel has shown great performance on glucose-responsive insulin release in our previous report.2 This hydrogel has a dual-network structure that consists of a chemical-crosslinked network among DexG molecules and a physical-crosslinked network between DexG and Con A. The chemical-crosslinked network contributes to the mechanical support, which may enable a long service life of the sensor, and the physical-crosslinked network dominates the reversible binding process of Con A and glucose. The network composition of the DexG-Con A hydrogel can be easily adjusted to meet the requirements for sensor design. The coplanar electrode is another distinctive factor of this sensor for its one-access side, which can couple with the responsive hydrogel. Kapton was used as the substrate to form an interdigital carbon electrode by direct laser writing. It is a rapid, inexpensive way to obtain carbon electrodes and can obviate the time-consuming lithography process.26–29 The dielectric property of the DexG-Con A hydrogel changes accordingly with the change of the environmental glucose concentration, and the signal change can be measured through ICEs, transducing the concentration signal into capacitance signal. Experimental results demonstrate that in a glucose concentration range of 0–30 mM, the proposed hydrogel sensor has the ability to measure the glucose level in a repeatable and reversible manner, showing a short responsive time of less than 2 min and has a high sensitivity of 8.81 pF mM−1 at a glucose range of 0–6 mM. Owing to its simple fabrication process, low-cost and high performance, the proposed glucose sensor has great potential on batch production for continuous glucose monitoring application.
Materials and methods
Materials
Concanavalin A (Con A, extracted from Jack Bean, pre-activated, Mw = 102 kDa, containing Ca2+ and Mn2+) was purchased from Medicago Inc. (Canada). Polyethylene glycol (600) diamethacrylate (PEGDMA) was obtained from TCI, Inc. (USA). Dextran (Mw = 70 kDa), glycidyl methacrylate (GMA), lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), tris(hydroxymethyl) aminomethane (Tris), HCl, D-glucose, D-fructose, D-galactose, lactate, and ascorbic acid were purchased from Sigma-Aldrich (USA). All of the regents were used as received. Kapton polyimide was obtained from Shenzhen Changdasheng Electronic Co., Ltd (China). DexG was synthesized by the ring-opening reaction of GMA with dextran according to our previous studies.23 The obtained derivative with 20.6% of substitution (DexG T70-20%) was used in the following experiments.Fabrication of ICEs by laser direct writing
Interdigital is a geometric structure applied in various sensor and transducer designs.30 Due to the one-side access structure, ICEs can easily carry the hydrogel to conduct a series of experiments. In this study, a laser direct writing equipment (Anhui Kejin Trading Co., Ltd) with the wavelength of 450 nm was used for carbonizing the surface of the polyimide (PI) film to produce the ICEs (8 mm × 12 mm, 6 digits with 0.8 mm of spacing). First, the surface of the PI film was cleaned by pure alcohol. Then, the PI film was loaded into the laser engraving equipment for processing (see Fig. S1†). The laser power for the fabrication was 4.9 W.Fabrication of the enzyme-free capacitive glucose sensor
The glucose-responsive hydrogel was formed in situ on the ICEs. At first, a mixture containing a specific amount of about 100 mg mL−1 of DexG, 23 mg mL−1 of Con A, 10 wt% of PEGDMA and 3 wt% of photoinitiator LAP was dissolved in a buffer solution (tris-HCl with pH 7.4), and then subjected to ultrasonic vibration at room temperature for 2 min. Next, a certain amount (30 μL) of the obtained solution was pipetted into a cuboid chamber (8 mm × 8 mm × 0.5 mm) enclosing the digits’ area of ICEs. After that, the device was placed in a dark box irradiated by a 365 nm LED UV light source, at 106 mW surface light intensity, for about 60 s. A thin hydrogel was formed right onto the ICEs, and fitted well with the finger boundaries. The obtained sensor was further immersed in the buffer solution (tris-HCl, pH 7.4) to protect the hydrogel and kept responsive for glucose (see Fig. S1†).Characterization of the ICEs
A scanning electron microscope (SEM) was used to observe the morphology of ICEs by a Hitachi S-3400 equipment (Hitachi, Japan). Raman spectroscopy was applied to investigate the crystallinity of the carbonized Kapton film through a Micro-Raman Spectrometer (Renishaw, UK) at a laser wavelength of 532 nm. X-ray photoelectron spectroscopy (XPS) tests were conducted by a Thermo Fisher spectrometer ESCALAB 250Xi (Thermo Fisher, UK).Performance study of the enzyme-free capacitive glucose sensor
The sheet resistance of the prepared carbonized PI (10 × 10 mm2) was investigated by a ST2253 four-point probe meter (Suzhou Jingge Electronic Co., Ltd). The impedance and capacitance of the enzyme-free capacitive glucose sensor were investigated by an LCR Meter (IM3536 LCR METER HIOKI) at a given AC signal of 5 V at a sequence of frequency from 1 to 80 kHz. Measurements were performed in a glucose buffer solution (Tris-HCl, pH 7.4) with increasing concentration from 0 to 30 mM at room temperature. The measurement of the glucose-induced capacitance change was repeated in the same manner in triplicate at 30 kHz. In addition, ascorbic acid, galactose, lactate and fructose were added into 5 mM glucose buffer with a concentration of ten percent of glucose to measure the capacitance change of the sensor in a circumstance with disturbances. Furthermore, to examine the time-resolved glucose-sensing manner of the obtained sensor, it was positioned into a fluid channel that was coupled with a peristaltic pump to change the glucose concentrations (see Fig. S2†). The peristaltic pump flow was set to 5 mL min−1. The flow rate of the glucose buffer solution was about 1.59 cm min−1, which is within the velocity range of human tissue fluid (0–7 cm min−1).Results and discussion
Fabrication of the enzyme-free capacitive glucose sensor
The enzyme-free glucose sensor consists of the DexG-Con A hydrogel formed by UV curing and ICEs fabricated through a UV-laser-based direct writing process. As shown in Fig. 1a, the DexG-Con A hydrogel was coated right onto the ICEs, and the enzyme-free glucose sensor was positioned in a fluid chamber for the detection of the concentration-variable glucose solutions. The DexG-Con A hydrogel as glucose-responsive element has good biocompatibility, rapid responsibility and easily adjusted network structure. Thus, this sensor has a robust performance, wide sensing range, and may work for implantable application. Compared to other capacitive glucose sensors, a laser direct writing technique is employed as an efficient and low-cost way to prepare ICEs. The obtained ICEs can be easily coated with the hydrogel layer, and have good conductivity for the capacitive glucose sensor.The DexG-Con A hydrogel has a dual-network structure for glucose detection. As illustrated in Fig. 1b, the covalent bonding among the DexG molecules supplies mechanical support for the hydrogel, while the specific physical binding between Con A and DexG contributes to the glucose-responsiveness.2 The competitive binding between DexG-Con A and glucose (Glu)-Con A affects the structure of the DexG-Con A network, and thus induces volume and permittivity change of the hydrogel. Furthermore, the binding process can be expressed by eqn (1) and (2), according to the ligand competition theory:2,31
| ConA + DexG ⇌ ConA-DexG | (1) |
| ConA + Glu ⇌ ConA-Glu | (2) |
As shown in Fig. 1c, when the glucose concentration increases, the Con A molecules have the priority to bind with glucose rather than DexG, causing the equilibrium of eqn (2) to shift towards the right side. At the same time, the equilibrium of eqn (1) shifts towards the left side. This leads to the expansion of the DexG-Con A network, and thus the swelling and permittivity change of the hydrogel. On the contrary, the hydrogel responds when the glucose concentration decreases.
Most of all, the permittivity change of the hydrogel caused by the concentration change of glucose can be detected using the ICEs by measuring the capacitance and impedance variations. The complex permittivity (ε*) of a substance can be presented by the following equation:
| ε* = ε − jε′ | (3) |
Characterization of the ICEs
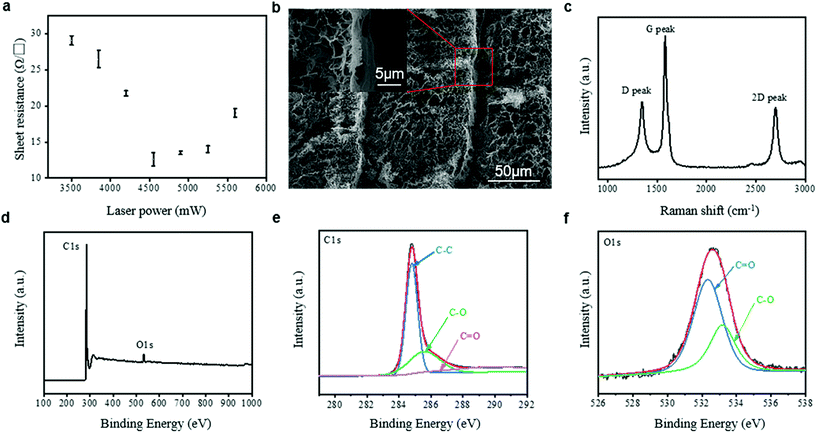
Carbon is a promising electrode material. Due to its high conductivity and porous structure, carbon performs well to improve the efficiency of the electron transfer.32,33 In our study, we used direct laser writing as a low-cost way to produce ICEs and investigated the optimum laser power. ICEs with high conductivity are desired for the capacitive glucose sensor. The sheet resistance of the obtained ICEs was optimized through the control of the laser power. Fig. 2a shows the sheet resistance of the ICEs at different laser powers. The variation of the sheet resistance at different laser powers could be separated into two segments. At the first segment from 3.5 to 4.75 W, the sheet resistance decreases with the increase of the laser power. Conversely, at the second segment from 4.75 to 6 W, the sheet resistance increases with the increment of the laser power. At the first segment, the increased laser power improves the crystallinity of the laser-generated carbon, thus contributing to the enhanced conductivity of the ICEs. With further increased laser power, it is observed that the formed carbon layer on the PI film is easily detached. This indicates that the carbon layer could be damaged by the increased laser power, thus contributing to the decreased conductivity of the ICEs. At the laser power of 4.55 W, the sheet resistance of the ICEs reaches the lowest value of 12.599 Ω □−1. The ICEs prepared at this condition will be employed for the fabrication of the enzyme-free capacitive glucose sensor.The surface morphology of the obtained carbon layer at the laser power of 4.55 W was studied, as illustrated in Fig. 2b. The laser ablation generated a porous carbon layer on the PI surface with a flake-like carbon species, which is similar to the laser generated structure reported by Wang et al.34Fig. 2c shows the Raman spectrum of the ICEs prepared at the laser power of 4.55 W. Three peaks located at 1350 (D-band), 1585 (G-band), and 2696 (2D-band) cm−1 were identified, which are due to the defects and disordered structures of graphitic carbons, sp2-hybridized graphitic carbon atoms, and the second order zone-boundary phonons of graphitic carbon, respectively.27 The value of ID/IG (the intensity ratio of the D peak and G peak) is 0.5039, indicating the good quality of the laser-generated carbon layer.35Fig. 2d illustrates the XPS spectrum of the carbon layer prepared at the laser power of 4.55 W. Compared to the O/C atomic ratio for the unablated PI film (∼18.3),36 the carbon generated by the laser has a much smaller O/C atomic ratio of 0.03. The decreased O/C atomic ratio after LDW could be ascribed to the gas release during the carbonization process.37 The curve-fitted XPS spectra for the C 1s and O 1s peaks are shown in Fig. 2e and f, respectively. The three peaks in the C 1s XPS spectrum are each assigned to C–C (284.8 eV), C–O (285.58 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.88 eV). The two peaks in the O 1s XPS spectrum each correspond to C
O (289.88 eV). The two peaks in the O 1s XPS spectrum each correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (532.34 eV) and C–O (533.2 eV). The spectrum in Fig. 2e has a relatively higher C–C peak than the C–O and C
O (532.34 eV) and C–O (533.2 eV). The spectrum in Fig. 2e has a relatively higher C–C peak than the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks. The high content of carbon ensures good electrical conductivity of the obtained ICEs.
O peaks. The high content of carbon ensures good electrical conductivity of the obtained ICEs.
Performance of the enzyme-free capacitive glucose sensor
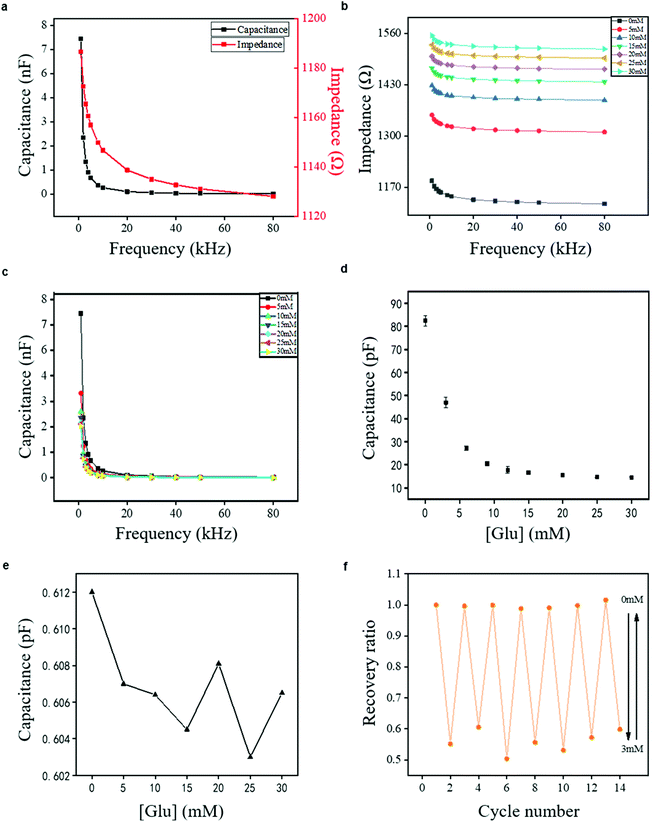
The frequency dependence of the capacitive sensor was investigated in response to various glucose concentrations in tris-HCl buffer (pH 7.4) at a set of frequency plots (1–80 kHz) by the LCR Meter. Fig. 3a shows that the capacitance and impendence decrease monotonously with the increase of frequency in the pure buffer solution without glucose. In addition, the dielectric properties of the hydrogel have an instantaneous change relative to the changing electric field, demonstrating the dielectric relaxation process of the hydrogel capacitor. This kind of process is created by the polarization effect, which reveals a shift of the electric charges from one equilibrium to another after a change of the electric field.38 The polarization effect inside the hydrogel may include electronic polarization, ionic polarization, dipolar polarization, counterion polarization, and interfacial polarization.39 The distortion of the electron clouds with the nucleus and the stretching of the atomic bonds can explain the electronic polarization and ionic polarization, while the redistribution of ions and reorientation of electrical dipoles are a reflection of the counterion polarization and dipolar polarization.40Fig. 3b and c show that the impedance and capacitance of the hydrogel sensor vary monotonically with increasing glucose concentration under each given voltage frequency. At a given low frequency, e.g., 1 kHz, the capacitance decreased from 7.08 to 2.23 nF. In addition, the impedance increased from 1186 to 1554 Ω as the glucose concentration changed within a wide range of 0–30 mM. The wide detection range of 0–30 mM is essential for glucose monitoring of diabetes patients. The same wide detection range has been reported in Zhang's work.41 The capacitance decrease tendency was also found at a typical higher frequency of 30 kHz, showing the decrease of capacitance from 69.0 to 25.2 pF and the increase of impedance from 1134 to 1524 Ω. A similar difference in capacitance (ΔC/C0 mM) was observed at different frequencies when the sensor was exposed to varied glucose concentrations (Fig. S3†). These results suggest that the change in the glucose concentration can be detected under different frequencies, which is of great potential for integration in a glucose detecting device. A high frequency of 30 kHz and a low frequency of 1 kHz were taken as examples for capacitance measurements in the following experiments. Determining which frequency should be chosen for the potential sensor device may depend on the convertor used for device integration.16
To test the glucose-responsive property of the proposed sensor, it was positioned into a series concentrations of glucose buffer solution, and the capacitance was measured (considering 30 kHz, for example) in response to the glucose concentration changes. The capacitance of the DexG-Con A hydrogel sensor decreased monotonically with glucose concentration increment, and then reached a saturated state at the glucose concentration of about 30 mM (Fig. 3d). Moreover, the sensitivity of the sensor was calculated by eqn (4), where ΔC and ΔGlu were defined as the capacitance and the concentration difference, respectively.
| Sensitivity = ΔC/ΔGlu | (4) |
Although the capacitance of the DexG-Con A hydrogel sensor showed a nonlinear dependence on the wide glucose concentration range of 0–30 mM, it displayed a partitioned linear dependence with varied sensitivity on each portion. The hydrogel sensor exhibited the highest sensitivity of 8.81 pF mM−1 at the glucose concentration range of 0–6 mM (R2 = 0.98), which showed great potential on the hypoglycemia diagnosis. Because the glucose level for sweat is 0.28–1.11 mM,42 this sensor may function well for glucose detection in sweat. The sensitivity of the DexG-Con A hydrogel sensor decreased with the increase of the glucose concentration above 6 mM. The sensitivity at the glucose concentration range of 9–15 mM was 0.640 pF mM−1 with an R square value of 0.98, and the sensitivity at the glucose concentration range of 15–25 mM was 0.142 pF mM−1 with an R square value of 0.95. A possible reason for the decreased sensitivity of the DexG-Con A hydrogel sensor lies in the reduced available binding sites on Con A with increased glucose concentration, causing the limitation of hydrogel swelling. Importantly, it can be found from Fig. 3e that the capacitance of ICEs is about two orders of magnitude lower than that of the whole sensor. In addition, it did not follow a monotonic pattern, indicating that the variation tendency of the sensor capacitance did not relate to the inherent nature of ICEs. As a sensor with a wide detecting range of glucose concentration, the proposed DexG-Con A sensor exhibits higher sensitivity compared to other capacitance glucose sensors, including two microsensors recorded by the Lin group with the sensitivity of 0.04 pF mM−1 at 0–5.56 mM (measured at 32 kHz)16 and 0.27 pF mM−1 at 0–2.22 mM (measured at 30 kHz).18 In addition, Fig. S4† reveals that the DexG-Con A hydrogel sensor has good stability in real-time detection over 1000 s (Fig. S4b†) and long-term working (Fig. S4a†).
The reversible response to glucose is one great advantage of both phenylboronic acid-based and Con A-based hydrogel sensors.18,25,43,44 To explore the reversible glucose response of the DexG-Con A hydrogel sensor, the capacitance in response to the glucose solution with repeated concentration change between 0 and 3 mM was recorded (see the real-time capacitance change in response to the glucose concentration change from 0 mM to 3 mM as an example in Fig. S5†), and the results are shown in Fig. 3f. The recovery ratio calculated by eqn (5) was used to demonstrate the recovery extent of the DexG-Con A hydrogel, where C represents the measured capacitance at the repeated glucose concentration change from 0 to 3 mM for seven cycles, respectively, and C0 mM represents the measured capacitance at the glucose concentration of 0 mM for the first cycle.
| recovery ratio = C/C0 mM | (5) |
The calculated value at the glucose concentration of 0 mM was among 0.987–1.016, while the value at the glucose concentration of 3 mM was decreased to 0.503–0.605. These results indicate that the glucose-binding of the developed hydrogel has good reversibility.
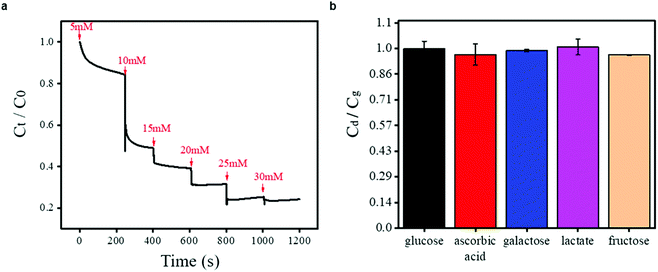
To verify the ability of the proposed DexG-Con A hydrogel sensor for continuous glucose sensing, an in vitro test platform was designed based on the fluid channel to perform continuous measurement. A peristaltic pump was used to exchange the glucose concentrations, and each solution change interval was less than 8 s, which was negligible for the whole experiment. The results are shown in Fig. 4a. As the glucose concentration changed from 5 to 30 mM, the value of Ct/C0 (where Ct represents the capacitance measured at time t and C0 represents the capacitance measured at 0 s, recorded at 1 kHz for example) of the DexG-Con A sensor decreased from 1 to 0.24. At a given concentration of glucose, the sensor responded fast and retained a relatively stable equilibrium. When the glucose concentration is 5 mM, the capacitance of the sensor can reach an equilibrium in less than 2 min. The faster response can be found with increased glucose level, owing to the higher osmotic pressure and faster binding reaction of Con A-glucose (according to eqn (2), a higher glucose concentration can cause faster equilibrium). A fast response has also been reported by the Zhang group in their hydrogel sensors.45,46 Although the response of the hydrogel sensors is usually slow, the thinness and the glucose-responsive principle of the DexG-Con A hydrogel may contribute to the fast change in response to different glucose concentrations. The physical binding between Con A and the sugar units directly induces the composition and conformational change of the hydrogel without any chemical reactions or ionization process.2
When the glucose sensor works in vivo, except for glucose molecule, other small molecules that exist in the interstitial fluid (including fructose, galactose, lactate, and ascorbic acid) may affect its capacitance. The physiological concentration of these disturbances is about one order of magnitude lower than the normal glucose level.16 In response to disturbances in the 5 mM glucose buffer solution, the capacitance of the DexG-Con A hydrogel sensor with their concentration of ten percent of glucose was measured (at 30 kHz), and is shown in Fig. 4b. Here, we use the capacitance ratio to indicate the glucose response in the presence of potential disturbances, which is calculated according to Cd/Cg, where Cg is the capacitance of the glucose solution (5 mM), and Cd is the capacitance of the glucose solution in the presence of different disturbances. The obtained capacitance ratio in response to the existence of ascorbic acid, galactose, lactate and fructose is 0.97, 0.99, 1.01, 0.96, respectively. This demonstrates that the DexG-Con A hydrogel sensor is resistant to the disturbances of small molecules in the interstitial fluid. These results indicate that the DexG-Con A hydrogel sensor is promising for implantable devices.
Conclusions
In this study, we designed a new hydrogel-based glucose sensor comprising a dual-network DexG/Con A hydrogel and a coplanar interdigital carbon electrode. The glucose-sensing hydrogel was built by UV curing of DexG and Con A. When the glucose molecules permeate into the hydrogel, they bind with Con A, inducing a change of the hydrogel networks. A laser was applied to pattern an interdigital capacitor for converting the change of a hydrogel network structure to the change of its dielectric property. Specifically, this method is non-enzymatic, non-catalytic and without any complex procedure for device fabrication. The experimental results showed that this hydrogel sensor had a wide sensing frequency of 1–80 kHz reflected by impedance and capacitance at a glucose concentration of 0–30 mM. In addition, it had a high sensitivity under a glucose concentration of 6 mM. The glucose response signal was reversible and the sensor performed well under real-time detection, which suggested that it would work for continuous glucose monitoring. Some disturbances in the blood and interstitial fluid had little influence on the sensor signal, and the influence could potentially be overcome by using a multi-sensor array. All materials that we used for the sensor design are biocompatible, indicating that the proposed sensor has great potential to be integrated in an implantable device.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to gratefully acknowledge the financial support by the Shanghai Science and Technology Innovation Program (No. 19441909600), the Opening project of Shanghai Key Laboratory of Orthopedic Implant (KFKT2019001) and the National Natural Science Foundation of China (Grant No. 51705154).References
- V. Gingras, N. Taleb, A. Roy-Fleming, L. Legault and R. Rabasa-Lhoret, Diabetes, Obes. Metab., 2018, 20, 245–256 CrossRef CAS
.
- R. Yin, M. Bai, J. He, J. Nie and W. Zhang, Int. J. Biol. Macromol., 2019, 124, 724–732 CrossRef CAS
.
- H. Thabit and R. Hovorka, Diabetologia, 2016, 59, 1795–1805 CrossRef CAS
.
- S. Wustoni, A. Savva, R. Sun, E. Bihar and S. Inal, Adv. Mater. Interfaces, 2018, 6, 1800928 CrossRef
.
- H. Al-Sagur, S. Komathi, M. A. Khan, A. G. Gurek and A. Hassan, Biosens. Bioelectron., 2017, 92, 638–645 CrossRef CAS
.
- X. Cheng, B. Wang, Y. Zhao, H. Hojaiji, S. Lin, R. Shih, H. Lin, S. Tamayosa, B. Ham, P. Stout, K. Salahi, Z. Wang, C. Zhao, J. Tan and S. Emaminejad, Adv. Funct. Mater., 2019, 30, 1908507 CrossRef
.
- J. Siegrist, T. Kazarian, C. Ensor, S. Joel, M. Madou, P. Wang and S. Daunert, Sens. Actuators, B, 2010, 149, 51–58 CrossRef CAS
.
- Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang and Y. Lei, Biosens. Bioelectron., 2010, 26, 542–548 CrossRef CAS
.
- D. W. Hwang, S. Lee, M. Seo and T. D. Chung, Anal. Chim. Acta, 2018, 1033, 1–34 CrossRef CAS
.
- S. Mondal, R. Madhuri and P. K. Sharma, J. Mater. Chem. C, 2017, 5, 6497–6505 RSC
.
- H.-W. Chang, Y.-C. Tsai, C.-W. Cheng, C.-Y. Lin and P.-H. Wu, Sens. Actuators, B, 2013, 183, 34–39 CrossRef CAS
.
- X. Niu, M. Lan, H. Zhao and C. Chen, Anal. Chem., 2013, 85, 3561–3569 CrossRef CAS
.
- V. Gingras, N. Taleb, A. Roy-Fleming, L. Legault and R. Rabasa-Lhoret, Diabetes, Obes. Metab., 2018, 20, 245–256 CrossRef CAS
.
- I. Tokarev and S. Minko, Soft Matter, 2009, 5, 511–524 RSC
.
- X. Huang, S. Li, J. Schultz, Q. Wang and Q. Lin, J. Microelectromech. Syst., 2009, 18, 1246–1254 CAS
.
- X. Huang, C. Leduc, Y. Ravussin, S. Li, E. Davis, B. Song, D. Li, K. Xu, D. Accili, Q. Wang, R. Leibel and Q. Lin, Lab Chip, 2014, 14, 294–301 RSC
.
- M. Elsherif, M. U. Hassan, A. K. Yetisen and H. Butt, Biosens. Bioelectron., 2019, 137, 25–32 CrossRef CAS
.
- J. Shang, J. Yan, Z. Zhang, X. Huang, P. Maturavongsadit, B. Song, Y. Jia, T. Ma, D. Li, K. Xu, Q. Wang and Q. Lin, Sens. Actuators, B, 2016, 237, 992–998 CrossRef CAS
.
- K. Aslan, J. R. Lakowicz and C. D. Geddes, Anal. Chim. Acta, 2004, 517, 139–144 CrossRef CAS
.
- B. Li, A. Yu and G. Lai, Carbon, 2018, 127, 202–208 CrossRef CAS
.
- T. Hoang, B. Stokke, U. Hanke, A. Johannessen and E. Johannessen, Appl. Sci., 2019, 9, 318–333 CrossRef CAS
.
- R. Yin, K. Wang, S. Du, L. Chen, J. Nie and W. Zhang, Carbohydr. Polym., 2014, 103, 369–376 CrossRef CAS
.
- M. Bai, J. He, L. Kang, J. Nie and R. Yin, Int. J. Biol. Macromol., 2018, 113, 889–899 CrossRef CAS
.
- R. Ballerstadt and J. S. Schultz, Anal. Chem., 2000, 72, 4185–4192 CrossRef CAS
.
- S. H. Paek, I. H. Cho, D. H. Kim, J. W. Jeon, G. S. Lim and S. H. Paek, Biosens. Bioelectron., 2013, 40, 38–44 CrossRef CAS
.
- M. Lobry, D. Lahem, M. Loyez, M. Debliquy, K. Chah, M. David and C. Caucheteur, Biosens. Bioelectron., 2019, 12, 111506 CrossRef
.
- S. Luo, P. T. Hoang and T. Liu, Carbon, 2016, 96, 522–531 CrossRef CAS
.
- M. F. EI-Kady and R. B. Kaner, ACS Nano, 2014, 8, 8725–8729 CrossRef
.
- Y. Gao, Q. Li, R. Wu, J. Sha, Y. Lu and F. Xuan, Adv. Funct. Mater., 2019, 29, 1806786 CrossRef
.
- A. V. Mamishev, K. Sundara-Rajan, Y. Fumin, D. Yanqing and M. Zahn, Proc. IEEE, 2004, 92, 808–845 CAS
.
- B. W. Sigurskjold, Anal. Biochem., 2000, 277, 260–266 CrossRef CAS
.
- Z. Zhu, L. Garcia-Gancedo, A. J. Flewitt, F. Moussy, Y. Li and W. I. Milne, J. Chem. Technol. Biotechnol., 2012, 87, 256–262 CrossRef CAS
.
- C. Chen, X. L. Zhao, Z. H. Li, Z. G. Zhu, S. H. Qian and A. J. Flewitt, Sensors, 2017, 17, 182 CrossRef
.
- F. Wang, K. Wang, X. Dong, X. Mei, Z. Zhai, B. Zheng, J. Lv, W. Duan and W. Wang, Appl. Surf. Sci., 2017, 419, 893–900 CrossRef CAS
.
- H.-P. Cong, P. Wang, M. Gong and S.-H. Yu, Nano Energy, 2014, 3, 55–63 CrossRef CAS
.
- K. C. Yung, D. W. Zeng and T. M. Yue, Appl. Surf. Sci., 2001, 173, 193–202 CrossRef CAS
.
- X. Li, X. Lu and Q. Lu, Appl. Surf. Sci., 2007, 253, 3690–3695 CrossRef CAS
.
-
F. Kremer and A. Schönhals, Broadband dielectric measurement techniques (10–6 Hz to 1012 Hz), Springer, Berlin, 2012, vol. 2 Search PubMed
.
- K. R. Foster and H. P. Schwan, Crit. Rev. Biomed. Eng., 1989, 17, 25–104 CAS
.
- Y. Feldman, E. Polygalov, I. Ermolina, Y. Polevaya and B. Tsentsiper, Meas. Sci. Technol., 2001, 12, 1355 CrossRef CAS
.
- X. Wang, Q. Li, Y. Guan and Y. Zhang, Mater. Today Chem., 2016, 1–2, 7–14 CrossRef
.
- O. Mickelsen and A. Keys, J. Biol. Chem., 1943, 149, 479–490 CAS
.
- Q. Li, Y. Guan and Y. Zhang, Sens. Actuators, B, 2018, 272, 243–251 CrossRef CAS
.
- C. M. Daikuzono, C. Delaney, H. Tesfay, L. Florea, O. N. Oliveira, A. Morrin and D. Diamond, Analyst, 2017, 142, 1133–1139 RSC
.
- S. Jia, Z. Tang, Y. Guan and Y. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 14254–14258 CrossRef CAS
.
- X. Zhang, Y. Guan and Y. Zhang, Biomacromolecules, 2012, 13, 92–97 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0an01672a |
| ‡ Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |