New simple molecular fluorescent probes for rapid and highly selective sensing of hydrazine by aggregate-induced emission†
Abstract
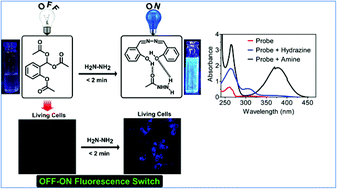
With an aim towards the design of efficient and straightforward fluorescent probes for hydrazine, the synthesis of (2-acetoxyaryl) methylene diacetate derivatives (1–4) was carried out by reacting substituted aromatic α-hydroxy aldehydes with acetyl chloride and sodium acetate in excellent yields. As a preliminary investigation, the ability of probe 1 was examined for the detection of substituted aliphatic and aromatic amines, amino acids, and other ions in Britton–Robinson buffer solution (50 mM, water/ethanol v/v of 99/1 at pH 7.4). Probe 1 selectively exhibited an intense blue fluorescence with hydrazine in less than 2 minutes, whereas light green or no fluorescence was noticed with substituted amines and amino acids. Among all the probes employed (1–4) in the present study, probes 1 and 2 were found efficient towards the rapid detection of hydrazine. Furthermore, the fluorescence sensing ability of probes 1 and 2 was tested not only under varying pH conditions but also by varying water-fraction from 0–99%. Moreover, the detection limits of hydrazine using 1 and 2 were found as 8.4 and 8.7 ppb, respectively, which is less than the acceptable limit as per the standards of the US Environment Protection Agency. In this contribution, the probes 1 and 2 demonstrate rapid, selective, sensitive, and ratiometric detection of highly toxic hydrazine by OFF–ON fluorescence switch in water samples as well as living cells.


 Please wait while we load your content...
Please wait while we load your content...