A handheld continuous-flow real-time fluorescence qPCR system with a PVC microreactor†
Abstract
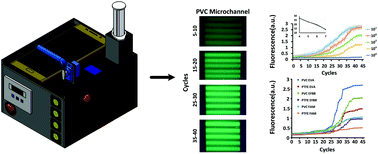
The polymerase chain reaction (PCR) has unique advantages of sensitivity, specificity and rapidity in pathogen detection, which makes it at the forefront of academia and application in molecular biology diagnosis. In this study, we proposed a hand-held real-time fluorescence qPCR system, which can be used for the quantitative analysis of nucleic acid molecules. For the first time, we use a PVC microreactor which improved the transmittance of the microreactor and made it easy to collect the fluorescence signal. In order to make it portable, the system adopted a passive syringe for sample injection and integrated temperature control and detection with a lithium battery for power supply. What's more, the fluorescence signal was captured by using a smartphone through an external automatic robotic arm. This real-time qPCR system can detect genomic DNA of the H7N9 avian influenza over four orders of magnitude of concentration from 107 to 104 copies per μL. In addition, it was verified that the fluorescence images obtained by this system were clearer than those obtained by a traditional system (using a PTFE spatial PCR microreactor) with two typical dyes and a probe tested—EvaGreen, SYBR Green and FAM.
- This article is part of the themed collections: Coronavirus articles - free to access collection, Coronavirus collection – Analytical and Smartphone-based sensors


 Please wait while we load your content...
Please wait while we load your content...