Open Access Article
Open Access ArticleClinical blood sampling for oxylipin analysis – effect of storage and pneumatic tube transport of blood on free and total oxylipin profile in human plasma and serum†
Katharina M.
Rund
a,
Fabian
Nolte
a,
Julian
Doricic
b,
Robert
Greite
b,
Sebastian
Schott
b,
Ralf
Lichtinghagen
c,
Faikah
Gueler
b and
Nils Helge
Schebb
 *a
*a
aChair of Food Chemistry, Faculty of Mathematics and Natural Sciences, University of Wuppertal, Wuppertal, Germany. E-mail: schebb@uni-wuppertal.de; Tel: +49 202-439-3457
bNephrology, Hannover Medical School, Hannover, Germany
cInstitute of Laboratory Medicine, Hannover Medical School, Hannover, Germany
First published on 3rd February 2020
Abstract
Quantitative analysis of oxylipins in blood samples is of increasing interest in clinical studies. However, storage after sampling and transport of blood might induce artificial changes in the apparent oxylipin profile due to ex vivo formation/degradation by autoxidation or enzymatic activity. In the present study we investigated the stability of free (i.e. non-esterified) and total oxylipins in EDTA-plasma and serum generated under clinical conditions assessing delays in sample processing and automated transportation: Free cytochrome P450 monooxygenase and 5-lipoxygenase (LOX) formed oxylipins as well as autoxidation products were marginally affected by storage of whole blood up to 4 h at 4 °C, while total (i.e. the sum of free and esterified) levels of these oxylipins were stable up to 24 h and following transport. Cyclooxygenase (COX) products (TxB2, 12-HHT) and 12-LOX derived hydroxy-fatty acids were prone to storage and transport induced changes due to platelet activation. Total oxylipin patterns were generally more stable than the concentration of free oxylipins. In serum, coagulation induced higher levels of COX and 12-LOX products showing a high inter-individual variability. Overall, our results indicate that total EDTA-plasma oxylipins are the most stable blood oxylipin marker for clinical samples. Here, storage of blood before further processing is acceptable for a period up to 24 hours at 4 °C. However, levels of platelet derived oxylipins should be interpreted with caution regarding potential ex vivo formation.
1. Introduction
Oxygenated polyunsaturated fatty acids (PUFA), i.e. eicosanoids and other oxylipins, are highly potent endogenous mediators of physiological and pathophysiological processes including regulation of endothelial function, blood pressure, thrombosis and inflammation.1–4Oxylipins are formed endogenously by conversion of PUFA via three major enzymatic pathways as well as non-enzymatic autoxidation leading to a multitude of oxygenated products from different PUFA precursors such as arachidonic acid (C20:4 n6), eicosapentaenoic acid (EPA, C20:5 n3) and docosahexaenoic acid (DHA, C22:6 n3): (I) conversion by cyclooxygenases (COX) results in formation of prostanoids and thromboxanes; (II) lipoxygenases (LOX) convert PUFA to regio- and stereoselective hydroperoxy-PUFA which can be reduced to hydroxy-PUFA or further converted to leukotrienes or multiple hydroxylated PUFA; and (III) action of cytochrome P450 monooxygenases (CYP) leads to formation of hydroxylated PUFA and epoxy-PUFA whereby the latter can be further hydrolyzed to vicinal dihydroxy-PUFA by soluble epoxide hydrolases.1,5,6 Besides regio- and stereoselective conversion by these enzymes, non-enzymatic radical mediated autoxidation gives rise to structurally similar oxygenated products with diverse regio- and stereochemistry such as isoprostanoids (IsoP), hydroxy-PUFA and epoxy-PUFA which also possess biological activity.7–10
Dysregulation of oxylipin formation is implicated among others in cardiovascular, immune or metabolic diseases11,12 and several pharmaceuticals act by modulating the oxylipin profile (e.g. non-steroidal anti-inflammatory drugs, anti-thrombotic drugs, the asthma drug zileuton).13–15 Considering the multitude and diversity of oxylipins as well as the different pathways involved in oxylipin formation the overall physiological effect might result rather from the general profile than from the levels of a single oxylipin.16,17 Therefore, comprehensive and reliable quantification of the oxylipin pattern may be valuable to evaluate the disease state or monitor the action of drugs.
For clinical diagnostics blood specimen, i.e. plasma or serum, are the most widely used biofluids reflecting the systemic condition of the donor. In blood, oxylipins occur in their free form, are associated with proteins, such as albumin,18,19 however the major part (>90%) – particularly hydroxy- and epoxy-PUFA as well as isoprostanoids – is esterified in cellular lipids (i.e. in blood cells) and in lipoproteins.20–22
Endogenous factors, such as sex,23 age,24 physical exercise,25,26 health status,27 intake of drugs13,15,28 or diet17,29 as well as variation in expression or genetic polymorphisms in enzymes30–33 impact the oxylipin profile of an individual. Moreover exogenous, pre-analytical factors relevant during collection, processing and storage of samples until analysis affect the oxylipin pattern,5 thus complicating its reliable interpretation.
In hospitals clinical blood samples are usually collected on a ward or in the emergency unit and are afterwards transported from the phlebotomy site to the clinical chemistry laboratory via transport systems such as pneumatic tube system transport (PTS) where centrifugation and generation of plasma or serum takes place.
The oxylipin pattern and its information about the physiology of the patient strongly depend upon the choice between serum or plasma during blood withdrawal. During serum generation the blood coagulation cascade is triggered which includes activation of platelets resulting in a massive increase of platelet derived oxylipins such as 12-LOX metabolites and thromboxanes.34,35 For plasma different anticoagulants during blood collection, such as EDTA, heparin or citrate are used. The type of anticoagulant affects the formation of oxylipins, particularly hydroxy-PUFA and platelet derived oxylipins (12-LOX and COX derived products).34,36 Therefore, blood sampling, i.e. the choice of the blood specimen, has to be carefully considered in clinical studies with respect to the analytes of interest and standardized within studies.
Following blood withdrawal, temperature and transitory storage time as well as transport to the clinical chemistry laboratory such as pneumatic tube system transport (PTS), are relevant pre-analytical factors which are hard to control in a clinical setting. Few studies investigated the influence of duration and temperature during whole blood storage prior further processing and revealed changes for some free, i.e. non-esterified oxylipins in the generated plasma especially after longer times (>30 min) at elevated temperatures (room temperature, RT).16,34,37 However, no data about the influence of typical sample handling in the clinic on the oxylipin pattern exist. This is especially necessary to evaluate the suitability and reliability of the oxylipin pattern in blood samples from clinical studies, as well as in samples collected from patients in emergency or intensive care units or from biobanks. Moreover, these data are an essential prerequisite to deduce the physiological role of changes in the oxylipin pattern in clinical studies as well as their suitability as biomarkers for disease.
In the present study we therefore investigated the impact of storage of blood samples for different time periods at 4 °C after collection, as well as the transfer from the ward to the clinical chemistry laboratory within a large hospital using PTS prior to the removal of cells (plasma or serum generation). For all conditions, levels of both, free and total oxylipins, i.e. after alkaline hydrolysis, were determined in EDTA-plasma and serum to evaluate if free or total oxylipins are more stable in plasma or serum. In order to deduce pathway specific effects a comprehensive set of oxylipins covering representative analytes derived from ARA, EPA and DHA from COX, LOX, CYP as well as non-enzymatic conversion was analyzed.
2. Experimental
2.1 Chemicals
Oxylipin and deuterated oxylipin standards were purchased from Cayman Chemicals (local distributor: Biomol, Hamburg, Germany). LC-MS-grade methanol (MeOH), LC-MS-grade acetonitrile (ACN), LC-MS-grade isopropanol, LC-MS-grade acetic acid were obtained from Fisher Scientific (Schwerte, Germany). Sodium acetate trihydrate, sodium hydrogen phosphate and n-hexane (HPLC grade) were obtained from Carl Roth (Karlsruhe, Germany) and potassium hydroxide (KOH, 85%) from Gruessing GmbH (Filsum, Germany). 2-(1-Thienyl)ethyl 3,4-dihydroxybenzylidenecyanoacetate (2-TEDC) was obtained from Santa Cruz. Ethyl acetate, phenylmethylsulfonyl fluoride (PMSF) and all other chemicals were purchased from Sigma Aldrich (Schnelldorf, Germany).2.2 Blood sampling and pre-analytical procedures
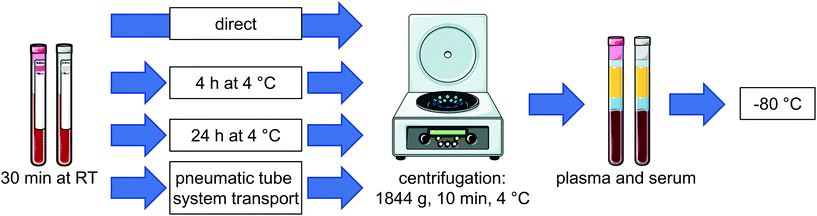
Venous blood samples were collected from 6 individual volunteers (4 females and 2 males, 23–56 years) by venipuncture in EDTA-monovettes (Sarstedt, Nümbrecht, Germany) and serum monovettes (Sarstedt, Nümbrecht Germany). Blood was collected at 10 am and no dietary restrictions were given. Each individual had breakfast before the blood sampling.From each individual plasma and serum samples were generated by centrifugation (4 °C, 10 min, 1844g, Thermo Scientific, Heraeus, Multifuge 3SR+) at 4 time points as described below and illustrated in Fig. 1: immediate, after 4 hours at 4 °C, after 24 hours at 4 °C and after pneumatic tube system transport (PTS) from the hospital ward to the clinical chemistry laboratory. For basal levels (t0) one plasma and serum monovette from each individual was centrifuged about 15 min after blood sampling. The supernatant, i.e. plasma or serum, was transferred to a cryotube and stored at −80 °C until analysis. To investigate the influence of storage on oxylipins, blood samples were maintained for about 30 min at room temperature reflecting the time blood sampling takes on a hospital ward. Afterwards monovettes from each individual were stored at 4 °C in the fridge for 4 hours or 24 hours until further processing to generate plasma or serum as described above. After 30 min of storage at room temperature an additional monovette was transferred by the PTS to the clinical chemistry laboratory where plasma and serum were generated in an analogous manner (ESI Fig. S1†). The pneumatic tube transport system of the Hannover Medical School comprises about 50 km pipe distance and works in suction operation. PTS transport of the samples ranged between 2 to 17 min. Overall samples sent via the PTS stayed approx. 1.5 to 3 h at room temperature until storage at −80 °C.
All procedures were conducted according to the guidelines laid down in the Declaration of Helsinki and approved by the ethic committee of the Hannover Medical School (MHH ethical approval 6895) and all volunteers gave their written informed consent.
To characterize effects on the sample state levels of lactate dehydrogenase (LDH) were determined in plasma samples from an additional sample set generated in an analogous manner using an Olympus analyzer (AU400) in an automated fashion according to the manufacturer's instruction (ESI Fig. S2†).
2.3 Extraction and quantification of oxylipins
Free oxylipins as well as total, i.e. free and esterified oxylipins were extracted from individual plasma and serum samples using anion exchange Bond Elut Certify II SPE cartridges (200 mg, 3 mL, Agilent, Waldbronn, Germany) as described with modifications.38Analysis of free oxylipins was carried out as described.39 In brief, to 500 μL plasma or serum 10 μL antioxidant solution (0.2 mg mL−1 BHT and EDTA, 100 μM cyclooxygenase inhibitor indomethacin, 100 μM of the soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB) in MeOH/water (50/50, v/v)), 10 μL of 250 mM protease inhibitor PMSF in isopropanol, 10 μL of 100 μM LOX inhibitor 2-TEDC in MeOH and 10 μL of an internal standard (IS) solution (containing 100 nM of 2H4-6-keto-PGF1α, 2H4-15-F2t-IsoP, 2H11-5(R,S)-5-F2t-IsoP, 2H4-PGE2, 2H4-PGD2, 2H4-TxB2, 2H4-LTB4, 2H4-9,10-DiHOME, 2H11-14,15-DiHETrE, 2H4-9-HODE, 2H8-5-HETE, 2H8-12-HETE, 2H6-20-HETE, 2H4-9(10)-EpOME, 2H11-14(15)-EpETrE) were added. Samples were diluted with 1 mL 1 M sodium acetate (pH 6, water/MeOH 95/5 v/v) and loaded on the preconditioned SPE cartridge.
Total, i.e. free and esterified oxylipins were extracted from 100 μL plasma or serum as described with slight modifications.40 After addition of 10 μL antioxidant solution and 10 μL IS solution, 400 μL isopropanol was added and samples were stored for 30 min at −80 °C for protein precipitation. After centrifugation the supernatant was hydrolyzed (300 μL 1.5 M KOH (75/25, MeOH/water, v/v)), immediately neutralized with 55 μL 50% acetic acid, diluted with 2 mL sodium phosphate buffer (pH 5.5) and extracted by SPE.
The extracts were analyzed in scheduled selected reaction monitoring mode following negative electrospray ionization by LC-MS/MS (QTRAP 6500, Sciex, Darmstadt, Germany).38,41
In parallel to the samples aliquots of pooled human quality control plasma (QC) were extracted using the same sample preparation procedure for free (n = 6) and total oxylipins (n = 7), respectively.
2.4 Data analysis
Oxylipins were quantified based on the analyte to corresponding IS area ratio using external calibration with linear least squares regression (1/x2 weighting) as described.38In order to evaluate the influence of storage or transport of blood on oxylipin levels in plasma and serum % differences vs. t0 (immediate sample processing) were calculated for each time point (tx) for each individual using the following formula: 100 × (conctx − conct0)/conct0. The relevance of change in the analyte concentration was evaluated by comparison with the acceptable change limit (ACL) calculated using ACL = 2.77 × RSDQC according to DIN ISO 5725-6.42 The factor 2.77 is based on 1.96 × 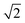 , where 1.96 is used to cover the 95% confidence interval for bi-directional changes and
, where 1.96 is used to cover the 95% confidence interval for bi-directional changes and 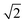 is used as the difference of two values is compared (at tx and t0). The relative standard deviation (RSD) was based on the RSD of quality control plasma samples (n = 6–7) analyzed in parallel with the samples (Table 1).
is used as the difference of two values is compared (at tx and t0). The relative standard deviation (RSD) was based on the RSD of quality control plasma samples (n = 6–7) analyzed in parallel with the samples (Table 1).
| Analyte | Free oxylipins | Total, i.e. free and esterified oxylipins | ||||||
|---|---|---|---|---|---|---|---|---|
| LLOQ [nM] | Mean ± SD [nM] | RSD [%] | ACL | LLOQ [nM] | Mean ± SD [nM] | RSD [%] | ACL | |
| a PGE2 and TxB2 are degraded during alkaline hydrolysis. | ||||||||
| PGE2 | 0.01 | 0.03 ± 0.004 | 17 | 48 | ||||
| TxB2 | 0.05 | 0.52 ± 0.06 | 12 | 32 | ||||
| 12-HHT | 0.05 | 0.53 ± 0.07 | 14 | 39 | 0.25 | 1.25 ± 0.13 | 9 | 26 |
| 12-HETE | 0.05 | 5.63 ± 0.89 | 16 | 44 | 0.25 | 8.48 ± 0.90 | 11 | 30 |
| 12-HEPE | 0.06 | 1.51 ± 0.28 | 18 | 51 | 0.31 | 1.14 ± 0.10 | 9 | 25 |
| 14-HDHA | 0.10 | 3.63 ± 0.55 | 15 | 42 | 0.50 | 2.98 ± 0.53 | 18 | 49 |
| 15-HETE | 0.13 | 1.14 ± 0.10 | 8 | 23 | 0.63 | 10.42 ± 1.50 | 14 | 40 |
| 15-HEPE | 0.06 | 0.20 ± 0.03 | 14 | 39 | 0.31 | 1.05 ± 0.15 | 14 | 39 |
| 17-HDHA | 0.20 | 0.89 ± 0.12 | 13 | 36 | 1.00 | 4.54 ± 0.75 | 17 | 46 |
| 5-HETE | 0.05 | 0.86 ± 0.09 | 11 | 29 | 0.25 | 17.77 ± 1.55 | 9 | 24 |
| 5-HEPE | 0.05 | 0.26 ± 0.03 | 13 | 36 | 0.25 | 2.69 ± 0.16 | 6 | 16 |
| 4-HDHA | 0.03 | 0.35 ± 0.03 | 7 | 21 | 0.13 | 3.62 ± 0.45 | 12 | 34 |
| 7-HDHA | 0.05 | 0.11 ± 0.02 | 21 | 58 | 0.50 | 2.47 ± 0.39 | 16 | 43 |
| 14(15)-EpETrE | 0.03 | 0.10 ± 0.01 | 9 | 24 | 0.25 | 54.46 ± 11.78 | 22 | 60 |
| 17(18)-EpETE | 0.10 | <LLOQ | 0.50 | 5.11 ± 0.88 | 17 | 48 | ||
| 19(20)-EpDPE | 0.05 | 0.31 ± 0.03 | 9 | 26 | 0.25 | 11.60 ± 2.06 | 18 | 49 |
| 14,15-DiHETrE | 0.01 | 0.64 ± 0.04 | 6 | 16 | 0.05 | 1.53 ± 0.16 | 11 | 29 |
| 17,18-DiHETE | 0.03 | 0.55 ± 0.06 | 11 | 31 | 0.13 | 0.80 ± 0.07 | 9 | 25 |
| 19,20-DiHDPE | 0.05 | 3.09 ± 0.17 | 5 | 15 | 0.25 | 3.77 ± 0.16 | 4 | 12 |
| 5(R,S)-5-F2t-IsoP | 0.05 | 0.11 ± 0.01 | 10 | 28 | 0.25 | 0.47 ± 0.05 | 11 | 31 |
| 11-HETE | 0.05 | 0.31 ± 0.02 | 7 | 20 | 0.25 | 6.42 ± 0.74 | 11 | 32 |
| 9-HETE | 0.25 | <LLOQ | 1.25 | 6.37 ± 0.77 | 12 | 34 | ||
| 18-HEPE | 0.10 | 0.42 ± 0.02 | 4 | 10 | 0.50 | 1.30 ± 0.15 | 12 | 33 |
Median concentrations of representative oxylipins with the interquartile range (25% percentil, 75% percentil) are given in the ESI (Tables S1 and S2†).
Data evaluation was carried out using GraphPad Prism version 6.01 for Windows (GraphPad Software, La Jolla California USA, http://www.graphpad.com).
3. Results
Oxylipins were analyzed by LC-MS/MS in plasma and serum from 6 individuals generated at four time points after blood collection in EDTA- and serum-tubes, respectively: (i) immediately; after storage at 4 °C for (ii) 4 hours or (iii) 24 hours; or (iv) after transfer via the pneumatic tube system (PTS) to the clinical chemistry laboratory (Fig. 1). At all conditions oxylipins were determined as free mediators (Fig. 2, ESI Fig. S3, S4, Table S1†) as well as after alkaline hydrolysis comprising both free and esterified oxylipins (Fig. 3, ESI Fig. S5, S6, Table S2†). In order to deduce effects of pre-analytical sample handling on the major enzymatic (COX, 5-LOX, 12-LOX, 15-LOX, CYP) and/or non-enzymatic formation pathways representative oxylipins were analyzed. In addition to the products formed from the conversion of ARA (Fig. 2 and 3) also respective counterparts derived from EPA and DHA (ESI Fig. S3–S6†) were evaluated to confirm observed pathway specific effects.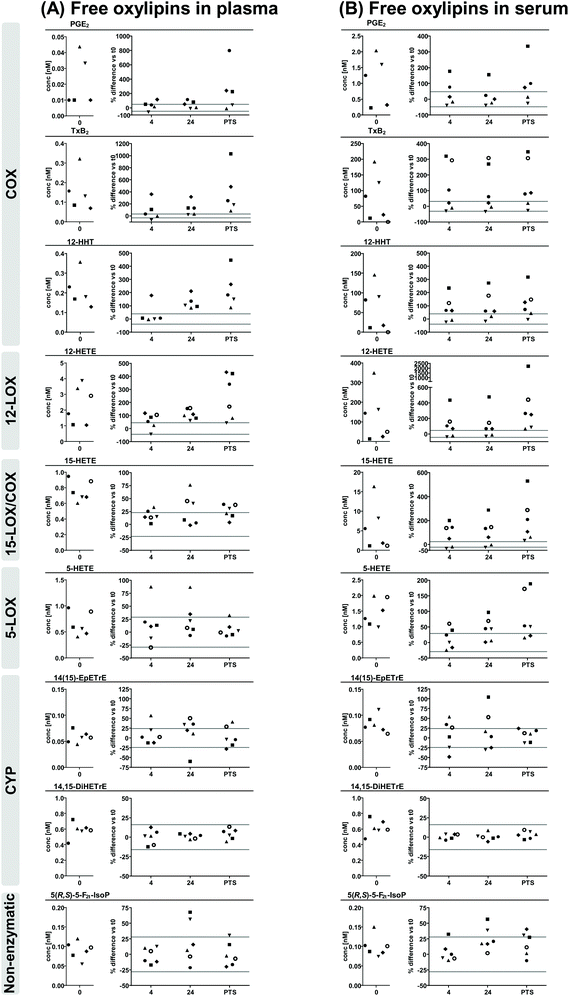 | ||
| Fig. 2 ARA derived free oxylipins in (A) plasma and (B) serum. Shown are individual concentrations (n = 6) of selected oxylipins from major formation pathways at t0 (immediate processing) and % difference vs. t0 after storage for 4 hours and 24 hours at 4 °C and after pneumatic tube system transport before centrifugation to generate plasma or serum. The different symbols represent samples from different individual human subjects. The grey lines indicate acceptable change limits calculated based on relative SD of quality control plasma (summarized in Table 1). | ||
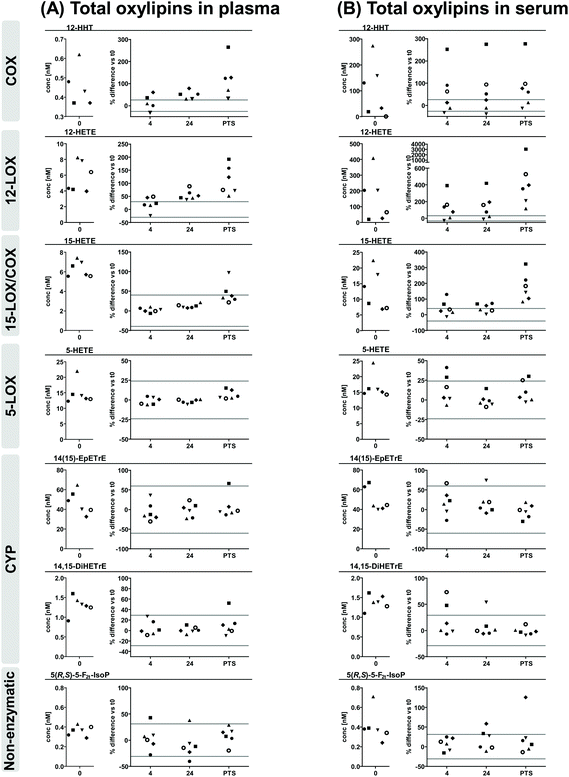 | ||
| Fig. 3 ARA derived total oxylipins in (A) plasma and (B) serum. Shown are individual concentrations (n = 6) of selected oxylipins from major formation pathways at t0 (immediate processing) and % difference vs. t0 after storage for 4 hours and 24 hours at 4 °C and after pneumatic tube system transport before centrifugation to generate plasma or serum. The different symbols represent samples from different individual human subjects. The grey lines indicate acceptable change limits calculated based on relative SD of quality control plasma (summarized in Table 1). | ||
3.1 Levels of oxylipins in plasma and serum after immediate processing
Comparing free oxylipin levels in plasma and serum after immediate processing (Fig. 2, ESI Fig. S3, S4†) revealed 2–3 orders of magnitude higher concentrations of COX-derived PGE2, TxB2 and 12-HHT in serum compared to plasma (21-125; 128-948; 68-500-fold, respectively). Similarly, also 12-LOX derived 12-HETE, 12-HEPE and 14-HDHA were massively increased (13-103; 2-73; 2-47-fold, respectively) in serum, while 15-LOX derived 15-HETE, 15-HEPE and 17-HDHA were elevated to a lesser extent (1-26; 1-6; 1-6-fold, respectively) and 5-LOX derived 5-HETE, 5-HEPE and 4-HDHA and 7-HDHA were unchanged or only slightly elevated (1-5; 1-2; 1-2-fold, respectively) in serum. In contrast, concentrations of CYP derived epoxy- and dihydroxy-PUFA were similar in plasma and serum consistent for ARA, EPA and DHA derived products. Levels of non-enzymatically formed ARA derived 5(R,S)-5-F2t-IsoP were comparable in plasma and serum.Regarding concentrations after immediate processing following alkaline hydrolysis (Fig. 3, ESI Fig. S5, S6†), levels of total oxylipins showed the same trend as their free counterparts when comparing plasma and serum: levels of epoxy- and dihydroxy-PUFA, as well as 5(R,S)-5-F2t-IsoP were comparable in plasma and serum. Serum levels of total 12-HHT were elevated to a similar extent (51-441-fold) compared to free 12-HHT. For hydroxy-PUFA differences between plasma and serum were moderate: levels of 12-LOX derived 12-HETE, 12-HEPE, 14-HDHA were clearly elevated (5-50; 2-14; 2-16-fold, respectively) while 15-LOX derived 15-HETE, 15-HEPE, 17-HDHA (1-3; 1-2; 1-3-fold, respectively) were same or only slightly higher in serum and levels of 5-LOX derived 5-HETE, 5-HEPE and 4-HDHA and 7-HDHA were similar in plasma and serum after hydrolysis.
Comparing levels of free and total oxylipins revealed similar concentrations for dihydroxy-PUFA while levels of hydroxy-PUFA, epoxy-PUFA and 5(R,S)-5-F2t-IsoP were massively higher after hydrolysis.
3.2 Analytical variability of oxylipins in plasma and serum
Stability of oxylipins in the differently stored and transported blood samples before processing to plasma and serum was evaluated based on changes in the apparent oxylipin levels in comparison to immediate processing and the “acceptable change limit” (ACL). The ACL covers the analytical variance including sample preparation and is based on the relative standard deviation of quality control plasma samples analyzed in parallel with the samples with similar concentrations (Table 1). For free oxylipins the ACL was below 35% for most analytes, except for PGE2 and 7-HDHA with concentrations near the LLOQ (48% and 58%, respectively) and for 12-LOX products (42–51%). Similarly, for total oxylipins the ACL was below 40% for most analytes, except epoxy-PUFA (48–60%) and DHA derived hydroxy-PUFA (43–49%).3.3 Stability of free oxylipins in plasma and serum
Regarding changes of free oxylipins in plasma after storage or PTS of blood samples, levels of 5(R,S)-5-F2t-IsoP, terminal dihydroxy-PUFA and epoxy-PUFA as well as 5-LOX products were stable at all investigated conditions with slight deviations for 5-LOX products in samples from a single individuum and for epoxy-PUFA in samples stored 24 h at 4 °C before processing. Changes of PGE2, TxB2 and 12-HHT were within the ACL for most samples after 4 h storage at 4 °C, while in samples stored for 24 h levels were clearly increased and after PTS further elevated with high inter-individual variance. 12-LOX metabolites showed a similar pattern with massive increases after PTS. With respect to 15-LOX metabolites, changes of 15-HETE and 17-HDHA were within the ACL in 4 h stored samples, clearly higher in 24 h stored samples, but only slightly elevated in PTS samples, while interestingly changes of 15-HEPE were within the ACL at all investigated conditions.In serum overall storage/transport-induced changes of free oxylipins were more pronounced. PGE2, TxB2 and 12-HHT showed a similar pattern in all stored and transported samples with high inter-individual variance being within the ACL for samples from 2–3 individuals, but massively higher for 2–4 individuals. For 12-LOX and 15-LOX metabolites changes were higher, though similar in 4 h and 24 h stored samples, but massively higher in PTS samples with high inter-individual variance. 5-LOX metabolites were within the ACL for most samples stored for 4 h, but exceeded the ACL in 24 h stored and PTS samples. While dihydroxy-PUFA were stable in all conditions, changes of epoxy-PUFA and 5(R,S)-5-F2t-IsoP were within the ACL in ≥50% of samples stored 4 h and after PTS, but were slightly higher than the ACL in 24 h stored blood samples.
3.4 Stability of total oxylipins in plasma and serum
Similar to free oxylipins, storage/transport-induced lower changes of total oxylipins in plasma compared to serum. However, the overall impact of sample handling was less pronounced in total levels compared to free oxylipins. In plasma and serum for all conditions changes of total 5-LOX metabolites, dihydroxy-PUFA, epoxy-PUFA as well as 5(R,S)-5-F2t-IsoP were within the respective ACL for ≥67% of the samples. 15-LOX-metabolites were stable in plasma of 4 h and 24 h stored samples, while in PTS samples slight increases were observed being most pronounced for DHA derived 17-HDHA. Deviations in serum were higher, slightly exceeding the ACL in 4 h and 24 h stored samples while being massively elevated in PTS samples with high inter-individual variance. The strongest influence of pre-processing handling was observed for 12-LOX metabolites, being more pronounced in serum than in plasma. While changes of 12-LOX metabolites in plasma were within the ACL in 4 h stored samples and slightly elevated in 24 h stored samples, in serum they already exceeded the ACL in ≥67% of all 4 h and 24 h stored samples. Changes in PTS samples were clearly higher than the ACL for plasma and serum, being however massively higher in serum with higher inter-individual variance. For total 12-HHT the extent and pattern of changes was similar as for free 12-HHT.4. Discussion
Targeted oxylipin metabolomics of clinical samples is increasingly becoming valuable in the evaluation of the disease state or for the discovery of disease biomarkers.5 However, blood sampling in clinical routine is often accompanied with uncontrolled transitory time intervals during collection and processing until further analysis and standardized handling of samples is hard to attain in the clinical setting. Efficient transport systems are established in large hospitals such as pneumatic tube system transport (PTS) to achieve fast sample delivery to the clinical chemistry laboratory. The influence of this “real life” blood sample handling has been previously described for hemolysis and clinical chemistry parameters,43,44 however, no information is available regarding its impact on the oxylipin pattern. Because of the oxidation-prone structure of oxylipins as well as the residual enzymatic activity in blood samples related to PUFA converting enzymes in blood cells, artificial formation and degradation of oxylipins during the pre-analytical workflow including collection, transport, processing, storage and preparation of samples cannot be excluded and may impact reliable interpretation of the analyzed oxylipin pattern. Therefore, we evaluated in the present study the effect of storage of whole blood samples at 4 °C for 4 h and 24 h as well as of the transport via the PTS prior centrifugation on the levels of a comprehensive set of free and total oxylipins in EDTA-plasma and serum covering oxylipins from all major formation pathways.4.1 Serum generation is associated with massive increase of free and total platelet derived oxylipins
In direct comparison of free oxylipin levels in directly processed plasma and serum we observed a massive increase of COX and 12-LOX derived products by 1–3 orders of magnitude in serum. Levels of 15-LOX products were also elevated however to a lesser extent, while 5-LOX derived metabolites were only slightly higher in serum, and CYP derived epoxy- and dihydroxy-PUFA were similar in plasma and serum. This is consistent with previous studies observing massive higher levels of free TxB2, 12-HHT and 12-HETE in serum compared to plasma.34,35,45 The observed increase in these eicosanoids is attributable to the intrinsic activation of the clotting cascade triggered by the contact of the contact system factor FXII which circulates in the blood with the negatively charged surfaces in the vacutainer used to generate serum during blood collection.46 Activation of this protease cascade ultimately leads to the activation of platelets and their main PUFA converting enzymes COX-1 and 12-LOX and subsequently to the formation of TxA2, 12-HHT (via COX-1) and 12-HETE (12-LOX) during coagulation.47,48 The observed increase in 15-HETE could also besides synthesis via 12/15-LOX which is under homeostatic conditions predominantly present in eosinophils49 related to activated platelets via COX-1 which have been shown to produce 15-HETE (predominantly the S-enantiomer) and 11-HETE.50 Consistently, 11-HETE showed a similar pattern (ESI, Fig. S7†). The slight elevation of 5-HETE levels in serum is also consistent with previous reports34,35 and the prevailing proportion of 5(S)-HETE35 suggests contribution of enzymatic formation via 5-LOX expressed in various leukocytes51 which have been implicated in the regulation of thrombosis.52Respective hydroxy-PUFA derived from EPA and DHA showed the same pattern as their ARA derived counterparts supporting the contribution of these enzymatic pathways to their formation during serum generation (ESI Fig. S3–S6†). However, these metabolites were elevated to a lesser extent reflecting the low levels of EPA and DHA in blood competing with ARA for their enzymatic conversion as human 12/15-LOX and 12-LOX enzymes have been shown to prefer DHA and EPA over ARA.53 The observation that levels of non-enzymatically formed 5(R,S)-5-F2t-IsoP were similar in plasma and serum suggests that autoxidative processes do not play a major role in freshly prepared serum. Similar only marginal alterations were seen for 9-HETE (ESI, Fig. S7†), which is discussed as autoxidation product.
The major portion of hydroxy- and epoxy-PUFA, as well as 5(R,S)-5-F2t-IsoP was found esterified in plasma and serum, because massive higher concentrations were observed after alkaline hydrolysis, consistent with earlier reports.20–22 Dihydroxy-PUFA were only present non-esterified with same apparent concentrations for free and total oxylipins. Since under alkaline conditions thromboxanes as well as β-hydroxy-keto-prostanoids, e.g. PGE2 and PGD2, are degraded,54 we used formation of 12-HHT as a surrogate for COX activity.47 The levels of 12-HHT were similar with and without hydrolysis yet massively higher in serum. Thus, based on 12-HHT also analysis of total oxylipins reflects increased COX activity due to platelet activation during serum generation.
Serum generation was also accompanied by an increase in total hydroxy-PUFA levels which was however less pronounced compared to the respective free hydroxy-PUFA. Even though it has been shown that eosinophilic 12/15-LOX directly form esterified 15-HETE in phospholipids49 and platelet 12-LOX derived hydroxy-PUFA are readily esterified contributing to the esterified pool,55 only a minor increase of 12- and 15-HETE (and respective EPA and DHA products) was observed in total oxylipins in comparison to their free levels. Esterified oxylipins in the cell free fraction of blood (i.e. serum and plasma) are thus hardly influenced by the activation of the coagulation cascade.
4.2 Plasma oxylipin profile is more stable than serum and total oxylipins are more stable than free oxylipins
The extended transitory storage of whole blood prior further processing to plasma or serum can cause formation and degradation of several oxylipins due to prolonged ex vivo contact with blood cells, i.e. erythrocytes, leukocytes or platelets, exhibiting enzymatic activity versus PUFA and oxylipins. In order to evaluate the impact of the transitory storage or PTS transport we used the ACL based on the methodological RSD obtained from quality control plasma samples analyzed in parallel42 which was in a similar range as previously reported for both, free34,56 and total oxylipins.40Free oxylipins in plasma from whole blood stored at 4 °C for 4 h remained almost stable (≥67% of the samples were within the ACL) with exception of samples from a few individuals showing higher deviations for 12-LOX and COX-1 derived products. After 24 h at 4 °C besides considerable increase in platelet-derived oxylipins also 15-LOX products were clearly increased. Though these results are consistent with a previous study reporting that free oxylipins in EDTA-whole blood are stable up to 2 h at 4 °C (ref. 34) or on wet ice,37 another study showed a clear decrease of some prostanoids (PGE2, PGF2α), hydroxy-PUFA (11- and 15-HETE) and epoxy-PUFA (14(15)- and 11(12)-EpETrE) in EDTA-whole blood already after 1 h on ice.16
In contrast to plasma, free oxylipins in serum of stored clotted whole blood were largely unstable. Especially platelet-derived (COX and 12-LOX) as well as 15-LOX products showed a strong increase with high inter-individual variance similar for 4 h and 24 h at 4 °C. 5-LOX derived products were stronger elevated after longer storage (24 h vs. 4 h). Consistently, La Frano et al. reported that a “freezing delay” of clotted whole blood dramatically elevated hydroxy-PUFA attributed to ongoing enzymatic processes in cooled whole blood.57
Apparent total oxylipin patterns were considerably more stable towards transitory storage of whole blood. However, the observed changes revealed a similar pattern as for free oxylipins: total plasma oxylipins were stable for 4 h at 4 °C and showed only elevation of platelet derived oxylipins after 24 h. In serum similarly to free oxylipins platelet derived oxylipins were massively increased after 4 h and 24 h, however increase in 15-LOX products was less pronounced and absent for 5-LOX products. These results suggest that transitory storage at 4 °C without removal of the blood cells or/and the blood clot led to enzymatic activity resulting from ex vivo activation of platelets. This leads to considerable artificial ex vivo formation of COX and 12-LOX products. While in plasma this can be ascribed to continuing enzymatic conversion related to the duration of storage, the high inter-individual variances in platelet-derived metabolites in serum which are similar at 4 h and 24 h indicate that these changes result rather from individual differences in the platelets and enzymatic activity of the blood coagulation cascade. One should note, that under all conditions the levels of free as well as total oxylipins of CYP derived dihydroxy-PUFA were stable and free levels of epoxy-PUFA showed only slight increases after 24 h. Moreover, the almost unchanged 5(R,S)-5-F2t-IsoP and 9-HETE levels indicate that only minor autoxidation takes place during the delay in sample processing.
4.3 Non-controlled blood transport amplifies formation of platelet derived (COX-1, 12-LOX) oxylipins
Automated pneumatic tube transport of blood caused in all samples a massive increase in 12-LOX and COX products with high inter-individual variances. While 15-LOX metabolites were only slightly and 5-LOX products barely affected in plasma, in serum PTS transport also led to a clear elevation of these hydroxy-PUFA. Again, these changes were more pronounced for free oxylipins. Consistent with previous studies observing decreased clotting time after PTS transport58,59 these observations suggest that during PTS transport platelets might be activated, e.g. due to shear stress or hemolysis induced by physical forces (shaking, rapid acceleration). Even though the used PTS has not been evaluated with regard to its impact on platelet function and different PTS might have varying effects, the observed increase in platelet derived COX and 12-LOX metabolites after PTS is likely attributable to enhanced platelet activation during PTS. Consistently, the observed higher variation of plasma LDH levels in samples undergoing PTS transport (RSD of relative difference 21%) compared to stored samples (RSD of relative difference 8–11%) indicates that PTS slightly induces hemolysis without being visibly noticeable (ESI, Fig. S2†).5. Conclusion
In the present study we investigated the impact of transitory storage as well as PTS transport of whole blood samples on the pattern of free and total oxylipins in plasma and serum with the aim to evaluate if free or total oxylipins are more stable in plasma or serum after whole blood handling in a clinical setting. Based on parallel analysis of oxylipins derived from ARA, EPA and DHA from all major formation pathways including COX, LOX, CYP as well as non-enzymatic conversion we could show: (1.) free oxylipins in plasma are stable up to 4 h at 4 °C and prolonged storage as well as sample transport led to increased levels of 12-LOX and COX products. (2.) The total oxylipin pattern in plasma and serum is less affected by transitory storage and transport. (3.) Blood coagulation, i.e. serum generation, leads to massively higher levels of free and total COX-1 and 12-LOX derived oxylipins due to ex vivo platelet activation. (4.) Serum samples showed higher storage and transport induced variability and inter-individual differences were more pronounced. Thus, with respect to clinical studies – where blood sampling cannot be tightly controlled – total plasma oxylipins seem to be the best analytical marker for the blood oxylipin pattern. While great caution with respect to artifact formation in interpretation of 12-LOX and COX metabolites is required, total levels of 5-LOX, CYP as well as the autoxidation product 5(R,S)-5-F2t-IsoP are stable with respect to blood handling conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Our work is supported by the Fonds der Chemischen Industrie to KR, the German Research Foundation (DFG, Grant SCHE 1801) and by a grant from the Federal Ministry of Education and Research (BMBF-No: 03INT502AB) to FG.References
- M. Gabbs, S. Leng, J. G. Devassy, M. Monirujjaman and H. M. Aukema, Adv. Nutr., 2015, 6, 513–540 CrossRef CAS PubMed.
- A. Konkel and W.-H. Schunck, Biochim. Biophys. Acta, 2011, 1814, 210–222 CrossRef CAS PubMed.
- J. Yeung, M. Hawley and M. Holinstat, J. Mol. Med., 2017, 95, 575–588 CrossRef CAS.
- E. A. Dennis and P. C. Norris, Nat. Rev. Immunol., 2015, 15, 511–523 CrossRef CAS PubMed.
- C. Gladine, A. I. Ostermann, J. W. Newman and N. H. Schebb, Free Radical Biol. Med., 2019, 144, 72–89 CrossRef CAS PubMed.
- M. W. Buczynski, D. S. Dumlao and E. A. Dennis, J. Lipid Res., 2009, 50, 1015–1038 CrossRef CAS PubMed.
- G. L. Milne, Q. Dai and L. J. Roberts II, Biochim. Biophys. Acta, 2015, 1851, 433–445 CrossRef CAS PubMed.
- H. Yin, L. Xu and N. A. Porter, Chem. Rev., 2011, 111, 5944–5972 CrossRef CAS PubMed.
- K. M. Rund, D. Heylmann, N. Seiwert, S. Wecklein, C. Oger, J.-M. Galano, T. Durand, R. Chen, F. Gueler, J. Fahrer, J. Bornhorst and N. H. Schebb, Prostaglandins Other Lipid Mediators, 2019, 144, 106334 CrossRef PubMed.
- H. Jiang, J. C. McGiff, J. Quilley, D. Sacerdoti, L. M. Reddy, J. R. Falck, F. Zhang, K. M. Lerea and P. Y. Wong, J. Biol. Chem., 2004, 279, 36412–36418 CrossRef CAS PubMed.
- V. Capra, M. Bäck, S. S. Barbieri, M. Camera, E. Tremoli and G. E. Rovati, Med. Res. Rev., 2013, 33, 364–438 CrossRef CAS.
- J. P. Hardwick, K. Eckman, Y. K. Lee, M. A. Abdelmegeed, A. Esterle, W. M. Chilian, J. Y. Chiang and B. J. Song, Adv. Pharmacol., 2013, 66, 157–266 CAS.
- K. Brune and P. Patrignani, J. Pain Res., 2015, 8, 105–118 CrossRef CAS PubMed.
- M. Crescente, L. Menke, M. V. Chan, P. C. Armstrong and T. D. Warner, Br. J. Pharmacol., 2019, 176, 988–999 CrossRef CAS.
- J. Song, X. Liu, T. S. Rao, L. Chang, M. J. Meehan, J. M. Blevitt, J. Wu, P. C. Dorrestein and M. E. Milla, J. Lipid Res., 2015, 56, 1492–1500 CrossRef CAS.
- I. Willenberg, A. I. Ostermann and N. H. Schebb, Anal. Bioanal. Chem., 2015, 407, 2675–2683 CrossRef CAS.
- A. I. Ostermann and N. H. Schebb, Food Funct., 2017, 8, 2355–2367 RSC.
- B. A. Ek-Von Mentzer, F. Zhang and J. A. Hamilton, J. Biol. Chem., 2001, 276, 15575–15580 CrossRef CAS.
- T. G. Brock, Lipids, 2008, 43, 161–169 CrossRef CAS.
- G. C. Shearer and J. W. Newman, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2008, 79, 215–222 CrossRef CAS.
- N. H. Schebb, A. I. Ostermann, J. Yang, B. D. Hammock, A. Hahn and J. P. Schuchardt, Prostaglandins Other Lipid Mediators, 2014, 113–115, 21–29 CrossRef CAS.
- J. D. Morrow, J. A. Awad, H. J. Boss, I. A. Blair and L. J. Roberts II, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 10721–10725 CrossRef CAS PubMed.
- S. Pace, L. Sautebin and O. Werz, Biochem. Pharmacol., 2017, 145, 1–11 CrossRef CAS.
- S. P. B. Caligiuri, M. Parikh, A. Stamenkovic, G. N. Pierce and H. M. Aukema, Am. J. Physiol.: Heart Circ. Physiol., 2017, 313, H903–H918 CrossRef.
- R. M. Giordano, J. W. Newman, T. L. Pedersen, M. I. Ramos and C. L. Stebbins, Int. J. Sport Nutr. Exercise Metab., 2011, 21, 471–479 CAS.
- X. Capo, M. Martorell, A. Sureda, J. A. Tur and A. Pons, J. Int. Soc. Sports Nutr., 2016, 13, 16 CrossRef CAS PubMed.
- A. M. Zivkovic, J. Yang, K. Georgi, C. Hegedus, M. L. Nording, A. O'Sullivan, J. B. German, R. J. Hogg, R. H. Weiss, C. Bay and B. D. Hammock, Metabolomics, 2012, 8, 1102–1113 CrossRef CAS PubMed.
- H. Gottschall, C. Schmöcker, D. Hartmann, N. Rohwer, K. Rund, L. Kutzner, F. Nolte, A. I. Ostermann, N. H. Schebb and K. H. Weylandt, J. Lipid Res., 2018, 59, 864–871 CrossRef CAS PubMed.
- D. D. Schramm, J. F. Wang, R. R. Holt, J. L. Ensunsa, J. L. Gonsalves, S. A. Lazarus, H. H. Schmitz, J. B. German and C. L. Keen, Am. J. Clin. Nutr., 2001, 73, 36–40 CrossRef CAS PubMed.
- C. C. Berthelot, S. G. Kamita, R. Sacchi, J. Yang, M. L. Nording, K. Georgi, C. Hegedus Karbowski, J. B. German, R. H. Weiss, R. J. Hogg, B. D. Hammock and A. M. Zivkovic, PLoS One, 2015, 10, e0144996 CrossRef PubMed.
- S. Ross, J. Eikelboom, S. S. Anand, N. Eriksson, H. C. Gerstein, S. Mehta, S. J. Connolly, L. Rose, P. M. Ridker, L. Wallentin, D. I. Chasman, S. Yusuf and G. Pare, Eur. Heart J., 2014, 35, 2242–2248 CrossRef CAS PubMed.
- C. B. Stephensen, P. Armstrong, J. W. Newman, T. L. Pedersen, J. Legault, G. U. Schuster, D. Kelley, S. Vikman, J. Hartiala, R. Nassir, M. F. Seldin and H. Allayee, J. Lipid Res., 2011, 52, 991–1003 CrossRef CAS PubMed.
- B. N. Zordoky and A. O. El-Kadi, Pharmacol. Ther., 2010, 125, 446–463 CrossRef CAS PubMed.
- J. Dorow, S. Becker, L. Kortz, J. Thiery, S. Hauschildt and U. Ceglarek, Biopreserv. Biobanking, 2016, 14, 107–113 CrossRef CAS PubMed.
- L. L. Mazaleuskaya, A. Salamatipour, D. Sarantopoulou, L. Weng, G. A. FitzGerald, I. A. Blair and C. Mesaros, J. Lipid Res., 2018, 59, 564–575 CrossRef CAS PubMed.
- H. S. Jonasdottir, H. Brouwers, R. E. M. Toes, A. Ioan-Facsinay and M. Giera, Biochim. Biophys. Acta, 2018, 1863, 1511–1522 CrossRef CAS PubMed.
- C. E. Ramsden, Z.-X. Yuan, M. S. Horowitz, D. Zamora, S. F. Majchrzak-Hong, B. S. Muhlhausler, A. Y. Taha, M. Makrides and R. A. Gibson, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2019, 150, 31–37 CrossRef CAS PubMed.
- K. M. Rund, A. I. Ostermann, L. Kutzner, J.-M. Galano, C. Oger, C. Vigor, S. Wecklein, N. Seiwert, T. Durand and N. H. Schebb, Anal. Chim. Acta, 2018, 1037, 63–74 CrossRef CAS PubMed.
- T. Greupner, L. Kutzner, F. Nolte, A. Strangmann, H. Kohrs, A. Hahn, N. H. Schebb and J. P. Schuchardt, Food Funct., 2018, 9, 1587–1600 RSC.
- A. I. Ostermann, E. Koch, K. M. Rund, L. Kutzner, M. Mainka and N. H. Schebb, Prostaglandins Other Lipid Mediators, 2019, 146, 106384 CrossRef PubMed.
- L. Kutzner, K. M. Rund, A. I. Ostermann, N. M. Hartung, J.-M. Galano, L. Balas, T. Durand, M. S. Balzer, S. David and N. H. Schebb, Front. Pharmacol., 2019, 10, 169 CrossRef CAS PubMed.
- DIN ISO 5725-6:2002-08, Accuracy (trueness and precision) of measurement methods and results – Part 6: Use in practice of accuracy values (ISO 5725-6:1994 including Technical Corrigendum 1:2001), Prepared by the Technical Comittee ISO/TC69, Applications of statistical methods, 2002, DOI:10.31030/9254896.
- R. Sodi, S. M. Darn and A. Stott, Ann. Clin. Biochem., 2004, 41, 237–240 CrossRef PubMed.
- J. Koessler, A. L. Kobsar, K. Brunner, H. Stolz, B. Dossler, U. Walter and U. Steigerwald, Clin. Chem. Lab. Med., 2011, 49, 1379–1382 CAS.
- M. Ishikawa, Y. Tajima, M. Murayama, Y. Senoo, K. Maekawa and Y. Saito, Biol. Pharm. Bull., 2013, 36, 682–685 CrossRef CAS PubMed.
- C. Maas, C. Oschatz and T. Renné, Semin. Thromb. Hemostasis, 2011, 37, 375–381 CrossRef CAS PubMed.
- T. Matsunobu, T. Okuno, C. Yokoyama and T. Yokomizo, J. Lipid Res., 2013, 54, 2979–2987 CrossRef CAS PubMed.
- V. B. O'Donnell, R. C. Murphy and S. P. Watson, Circ. Res., 2014, 114, 1185–1203 CrossRef PubMed.
- S. Uderhardt, J. A. Ackermann, T. Fillep, V. J. Hammond, J. Willeit, P. Santer, M. Mayr, M. Biburger, M. Miller, K. R. Zellner, K. Stark, A. Zarbock, J. Rossaint, I. Schubert, D. Mielenz, B. Dietel, D. Raaz-Schrauder, C. Ay, T. Gremmel, J. Thaler, C. Heim, M. Herrmann, P. W. Collins, G. Schabbauer, N. Mackman, D. Voehringer, J. L. Nadler, J. J. Lee, S. Massberg, M. Rauh, S. Kiechl, G. Schett, V. B. O'Donnell and G. Krönke, J. Exp. Med., 2017, 214, 2121–2138 CrossRef CAS PubMed.
- F. Rauzi, N. S. Kirkby, M. L. Edin, J. Whiteford, D. C. Zeldin, J. A. Mitchell and T. D. Warner, FASEB J., 2016, 30, 4256–4266 CrossRef CAS PubMed.
- J. Z. Haeggstrom and C. D. Funk, Chem. Rev., 2011, 111, 5866–5898 CrossRef PubMed.
- L. L. Swystun and P. C. Liaw, Blood, 2016, 128, 753–762 CrossRef CAS PubMed.
- L. Kutzner, K. Goloshchapova, D. Heydeck, S. Stehling, H. Kuhn and N. H. Schebb, Biochim. Biophys. Acta, 2017, 1862, 666–675 CrossRef CAS PubMed.
- C. Gladine, J. W. Newman, T. Durand, T. L. Pedersen, J.-M. Galano, C. Demougeot, O. Berdeaux, E. Pujos-Guillot, A. Mazur and B. Comte, PLoS One, 2014, 9, e89393 CrossRef PubMed.
- V. J. Hammond and V. B. O'Donnell, Biochim. Biophys. Acta, 2012, 1818, 2403–2412 CrossRef CAS PubMed.
- A. I. Ostermann, T. Greupner, L. Kutzner, N. M. Hartung, A. Hahn, J. P. Schuchardt and N. H. Schebb, Anal. Methods, 2018, 10, 4935–4944 RSC.
- M. R. La Frano, S. L. Carmichael, C. Ma, M. Hardley, T. Shen, R. Wong, L. Rosales, K. Borkowski, T. L. Pedersen, G. M. Shaw, D. K. Stevenson, O. Fiehn and J. W. Newman, Metabolomics, 2018, 14, 151 CrossRef PubMed.
- S. Thalén, I. Forsling, J. Eintrei, L. Söderblom and J. P. Antovic, Thromb. Res., 2013, 132, 77–80 CrossRef PubMed.
- O. Wallin, J. Soderberg, K. Grankvist, P. A. Jonsson and J. Hultdin, Clin. Chem. Lab. Med., 2008, 46, 1443–1449 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9an01880h |
| This journal is © The Royal Society of Chemistry 2020 |