Parallel [TG(GA)3]n-homoduplexes/thioflavin T: an intense and stable fluorescent indicator for label-free biosensing†
Qiang
Liu
a,
Shaochun
Jing
a,
Mei
Liu
b,
Yan
Jin
 a and
Baoxin
Li
a and
Baoxin
Li
 *a
*a
aSchool of Chemistry & Chemical Engineering, Key laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Shaanxi Normal University, Xi'an 710062, China. E-mail: libaoxin@snnu.edu.cn; Fax: +86-29-81530727
bCollege of Food Engineering and Nutritional Science, Shaanxi Normal University, Xi'an 710062, China
First published on 11th November 2019
Abstract
Different from the classical antiparallel DNA double-stranded structure, parallel DNA duplexes possess unique structures and potential biological functions. In this work, we found that the parallel DNA homoduplex from the [TG(GA)3]n sequence ([TG(GA)3]n-dsDNA) can dramatically enhance the fluorescence of thioflavin T (ThT), and the fluorescence enhancement is proportional to the number (n) of TG(GA)3 units in [TG(GA)3]n. Compared with the traditional G-quadruplex/ThT system, [TG(GA)3]n/ThT showed more stable and stronger fluorescence emission. In addition, coupled with an isothermal exponential amplification reaction, [TG(GA)3]3/ThT was used as a label-free fluorescent probe to detect microRNA, and the [TG(GA)3]3/ThT probe exhibited higher sensitivity than the G-quadruplex/ThT probe. This work provides a new paradigm to design label-free fluorescent biosensing/imaging systems.
Introduction
Thioflavin T (ThT) is a commercial cationic benzothiazole dye. Due to the intramolecular rotations of the benzothiazole ring and dimethylaniline ring around the C–C bond, free ThT exhibits extremely low fluorescence emission in aqueous solution. Upon binding to amyloid fibrils, ThT fluorescence enhances.1,2 So, ThT has become a common fluorescent indicator for in vitro quantification of amyloid formation, and is often used to measure the pharmacodynamics and kinetics of inhibitors for amyloid-related diseases.1–4 Recently, it has been reported that ThT could induce folding of G-rich DNA or RNA sequences into a G-quadruplex, and further bind with the G-quadruplex via end-stacking to significantly enhance its fluorescence emission.5,6 Based on the fluorescence properties, G-quadruplex/ThT is widely used as an indicator for constructing label-free fluorescent biosensing systems.7 The G-quadruplex/ThT-based sensing platform is simple, sensitive, and has been applied to detect DNA,5,8,9 microRNA,10,11 proteins,12,13 and other targets.14,15 G-quadruplexes show a rich structural polymorphism, such as parallel, antiparallel and mixed conformations.16 G-quadruplex conformations are sensitive to the stabilizing cation as well as to the molecular effect.17,18 One G-rich DNA strand can fold into different G-quadruplex conformations depending on the experimental conditions. One of the known examples is the human telomeric DNA strand (5′-AGG GTT AGG GTT AGG GTT AGG G-3′), which can adopt at least 10 different conformations;6 the human telomeric sequence folds exclusively into an antiparallel G-quadruplex in the presence of Na+,19,20 whereas K+ induces the folding of the human telomeric DNA into a mixed parallel and antiparallel G-quadruplex conformation.21,22 Different G-quadruplex topologies would produce distinctly different fluorescence enhancements of ThT.5 When different cations (such as K+, Na+ and NH4+) co-exist together, the fluorescence signal of G-quadruplex/ThT will be seriously disturbed, inevitably generating “false positive” results. Additionally, several small molecules can also induce the G-quadruplex to fold into different topology structures,23–25 which will also lead to changes in the fluorescence intensity of the G-quadruplex/ThT sensing system. It is obvious that the G-quadruplex/ThT fluorescent indicator is not very stable, which limits the wide application of the G-quadruplex/ThT sensing system.Different from classical antiparallel double-stranded DNA (dsDNA), parallel dsDNA represents a unique DNA duplex with both sugar-phosphate chains pointing in the same direction.26 Due to its special structure and potential biological function, parallel dsDNA has attracted more and more attention.27–30 Recently, Liu et al. reported that GA-containing parallel dsDNA could bind ThT to enhance its fluorescence.31 This work illustrated that GA-containing parallel dsDNA as a class of non-G-quadruplexes could improve ThT fluorescence, but the fluorescence of parallel dsDNA/ThT was lower than that of G-quadruplex/ThT.31 The Wang group found that introduction of a TG base at the 5′ end of the GA-containing parallel dsDNA could improve the fluorescence intensity of the parallel dsDNA/ThT.32 The researchers expect that parallel dsDNA/ThT can be used as a fluorescent indicator to construct DNA-based sensors. The GA-containing parallel dsDNA is homoduplex. One unit of parallel dsDNA/ThT needs two DNA strands, whereas one unit of G-quadruplex/ThT usually needs one strand. If the fluorescence intensity of parallel dsDNA/ThT is more than twice that of G-quadruplex/ThT, parallel dsDNA/ThT-based sensing is superior to G-quadruplex/ThT-based sensing in terms of sensitivity. However, the fluorescence of the reported parallel dsDNA/ThT was two-fold lower than that of G-quadruplex/ThT fluorescence.31,32
In this work, we studied in detail the effect of a GA-rich oligonucleotide sequence on the fluorescence intensity of parallel dsDNA/ThT. Interestingly, we found that the parallel DNA homoduplex formed from the [TG(GA)3]n sequence (denoted as [TG(GA)3]n-dsDNA) can dramatically enhance the fluorescence of ThT, and the fluorescence intensity of parallel [TG(GA)3]3-dsDNA/ThT is 2-fold higher than that of G-quadruplex/ThT. Moreover, we have systematically investigated the stability of parallel [TG(GA)3]3-dsDNA in the presence of different univalent cation species, and the results suggested that the fluorescence stability of parallel [TG(GA)3]3-dsDNA/ThT was superior to that of G-quadruplex/ThT. As proof-of-concept experiments, we combined the parallel [TG(GA)3]3-dsDNA/ThT fluorescence signal read-out with that of an isothermal exponential amplification reaction (EXPAR) to develop a label-free biosensor for microRNA (miRNA) detection. Compared with the G-quadruplex/ThT signal readout, the parallel [TG(GA)3]3-dsDNA/ThT system was more sensitive and stable. In a word, this work may not only open a novel avenue for ThT fluorescence enhancement, but also provide a stable fluorescent indicator for constructing DNA-based sensors and devices, which will be highly beneficial for the application of parallel [TG(GA)3]3-dsDNA/ThT in biosensing/imaging.
Experimental
Reagents and materials
All oligonucleotides used in this work were synthesized and HPLC-purified by Sangon Biotech Co., Ltd (Shanghai, China). The sequences of the oligonucleotide strands are listed in Table S1.† 100 μM oligonucleotide stock solutions were prepared in pH 7.4 Tris–HCl buffer (5 mM) and diluted to the desired concentrations with pH 7.4 Tris–HCl buffer. ThT was purchased from Sigma Chemical Co. (St Louis, USA). KCl and NaCl were obtained from Guangdong Guanghua Sci-Tech Co., Ltd (Beijing, China). MgCl2, NH4Cl and HCl were purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China). Vent (exo-) DNA polymerase and Nt·BstNBI nicking endonuclease were purchased from New England Biolabs (MA, USA). The mixture of deoxynucleotide triphosphates (dNTPs) was obtained from Sangon Bio. Eng. Tech. & Services Co., Ltd (Shanghai, China). 18 MΩ cm−1 ultrapure water was obtained using a Millipore water purification system (Billerica, MA, USA) and was used in all experiments.Fluorescence spectroscopic measurements
In this work, 5 mM Tris–HCl (pH 7.4, 20 mM MgCl2) was used as a buffer solution. ThT was mixed with [TG(GA)3]n or G4 DNA, and then the mixture was incubated at 90 °C for 5 min and gradually cooled to room temperature. Finally, the fluorescence emission spectra of the final solution were recorded using an F-4600 fluorometer (Hitachi, Tokyo, Japan). The fluorescence emission spectra were collected from 450 to 580 nm with an excitation wavelength of 425 nm. The PMT detector voltage was 700 V, and the slit widths of the excitation and emission were set at 10 nm. The fluorescence emission intensity was measured at 490 nm.Circular dichroism measurements
Circular dichroism (CD) spectroscopy was performed using a Circular Dichroism Spectrometer (Applied Photophysics Ltd, England, UK) at ca. 25 °C with an optical chamber with 0.1 cm path length. The bandwidth was set at 1.0 nm, and the CD spectra were recorded from 200 to 500 nm at a scanning speed of 200 nm min−1 with the response time of 0.5 s. All samples were prepared by adding 10 μL oligomers (1 × 10−4 M) and 10 μL ThT (1 × 10−3 M) to 180 μL pH 7.4 Tris–HCl buffer (5 mM). The reaction volumes of all the samples were 200 μL, and they were incubated at room temperature for one hour. Under the same conditions, three scans were accumulated, and their average values were taken.EXPAR biosensing strategy for microRNA detection
Firstly, 10 μL different concentrations of microRNA and 1 μL template (0.1 μM) were added into 39 μL nicking endonuclease buffer (pH 7.9 Tris–HCl (25 mM), 125 mM NaCl, 1.25 mM dithiothreitol and 12.5 mM MgCl2), then the above mixture was heated at 88 °C for 3 min, and allowed to cool naturally to room temperature (ca. 25 °C). Subsequently, 39 μL ThermoPol buffer (20 mM Tris–HCl, pH 8.8, 25 mM KCl, 25 mM (NH4)2SO4, 5 mM MgCl2, and 0.25% Triton X-100), 1 μL 25 mM dNTP, 5 μL of 2 U μL−1 Nt·BstNBI and 5 μL of 0.2 U μL−1 vent (exo-) polymerase were added into the above solution mixture. After incubation for 90 min at 55 °C, 50 μL of 2.5 μM ThT solution (5 mM Tris–HCl, 2.5 mM Mg2+ for the [TG(GA)3]3/ThT probe, while for the G-quadruplex/ThT probe, 25 mM Tris–HCl, 75 mM K+) was added into the system, and then incubated for 1 h at 25 °C. Finally, the fluorescence emission spectra of the final solution were recorded using an F-4600 fluorometer (Hitachi, Tokyo, Japan). The excitation wavelength was set at 425 nm, and the slit widths of the excitation and emission were set at 10 nm. The fluorescence intensity at 490 nm was used to quantify the target microRNA concentration.Results and discussion
Fluorescence enhancement of ThT by the parallel [TG(GA)3]n-homoduplex
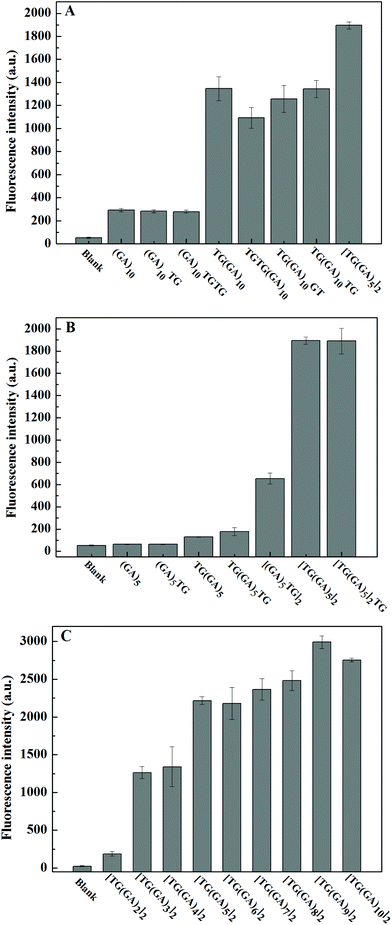
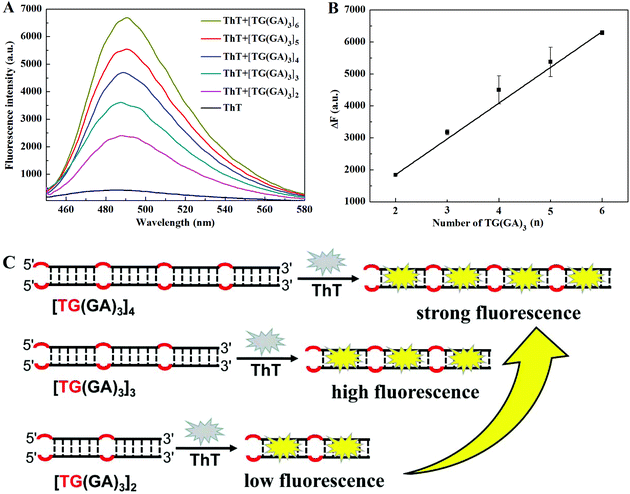
DNA sequences often play a decisive role in small molecular interactions. GA-containing parallel dsDNA can bind and enhance ThT fluorescence,31 and Wang and co-workers found that “TG” bases at the 5′ end could improve the enhancement effect of (GA)10-dsDNA.32 Inspired by their work, we designed several different (GA)10 strands, including one or two “TG” bases at the 5′ end and/or the 3′ end, or in the middle of the (GA)10 strand, and explored their enhancement effect on ThT fluorescence. As shown in Fig. 1A, the results indicated that only the “TG” bases at the 5′ end of the (GA)10 sequence could improve the enhancement of fluorescence intensity of ThT by GA-containing parallel dsDNA, which was consistent with the reported literature.32 More importantly, we found that, compared to TG(GA)10/ThT, the fluorescence intensity of [TG(GA)5]2/ThT increased about 36%, which suggests that the “TG” bases of the (GA)5 sequence may improve the enhancement of the fluorescence intensity of ThT. To verify this hypothesis, we designed a few different (GA)5 strands. As indicated in Fig. 1B, the fluorescence intensity of [TG(GA)5]2/ThT was about three times stronger than that of [(GA)5TG]2/ThT, which further confirmed that “TG” bases at the 5′ end is important for fluorescence enhancement. We studied the effect of [TG(GA)n]2 sequences with different lengths on ThT fluorescence. As shown in Fig. 1C, the enhancement efficiency increases with the increase in the number of “GA” bases (n) in parallel dsDNA; when the n value increases from 2 to 3, the fluorescence intensity sharply increases from 186 to 1263; above n > 3, the increase becomes rather slow. These results showed that from the perspective of the length of DNA, [TG(GA)3]2 may be a better sequence for enhancing ThT fluorescence. In order to continue exploring the enhancement effect of parallel dsDNA on ThT fluorescence, we also studied the effect of [TG(GA)3]n sequences with different lengths. As shown in Fig. 2A, the fluorescence intensity of [TG(GA)3]n/ThT gradually increases when the number of TG(GA)3 motifs (n) increases from 2 to 6, and there is a linear relationship between the enhanced fluorescence intensity (ΔF) and the number of TG(GA)3 motifs (n) (Fig. 2B). We also calculated the enhancement ratio for different lengths of [TG(GA)3]n/ThT, ΔF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔF5
ΔF5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔF4
ΔF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔF3
ΔF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔF2 = 5.9
ΔF2 = 5.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.1
5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.1
4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1
3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4, which is approximately equal to the theoretical value (6
1.4, which is approximately equal to the theoretical value (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Therefore, as indicated in Fig. 2C, we could reach two conclusions: (1) TG(GA)3 is the basic unit for binding ThT; (2) the fluorescence intensity of [TG(GA)3]n/ThT is proportional to the number of TG(GA)3 motifs. To confirm these conclusions, we compared the fluorescence intensity of [TG(GA)3]n/ThT at different concentrations. As indicated in Fig. S1A,† when the n value is constant, the fluorescence intensity of [TG(GA)3]n/ThT is proportional to the concentration of [TG(GA)3]n. For example, the [TG(GA)3]2 concentration increased from 1 × 10−7 M to 2 × 10−7 M, and the corresponding fluorescence intensity of [TG(GA)3]2/ThT also increased and doubled. Furthermore, we compared the fluorescence intensity of [TG(GA)3]n/ThT with different concentrations and different numbers of TG(GA)3 motifs (n). It can be seen from Fig. S1B† that the fluorescence intensities of [TG(GA)3]n/ThT are about the same when C × n (where C is the concentration of [TG(GA)3]n, and n is the number of TG(GA)3 motifs) is equal (such as, 1 × 4 = 2 × 2, 1 × 6 = 2 × 3 = 3 × 2). These results confirm that TG(GA)3 is the basic unit for binding ThT in the parallel [TG(GA)3]n-homoduplex. On the other hand, we measured the viscosity of the DNA solutions with different sequences and different concentrations (0.5 × 10−7 mol L−1, 0.7 × 10−7 mol L−1 and 1 × 10−7 mol L−1). The experimental results showed that the viscosity of the DNA solutions was almost the same as the viscosity of the solvent (H2O), and the viscosity of the DNA solutions did not change with changing DNA sequences and concentrations. So, the effect of solution viscosity on fluorescence could be ruled out in this system.
2). Therefore, as indicated in Fig. 2C, we could reach two conclusions: (1) TG(GA)3 is the basic unit for binding ThT; (2) the fluorescence intensity of [TG(GA)3]n/ThT is proportional to the number of TG(GA)3 motifs. To confirm these conclusions, we compared the fluorescence intensity of [TG(GA)3]n/ThT at different concentrations. As indicated in Fig. S1A,† when the n value is constant, the fluorescence intensity of [TG(GA)3]n/ThT is proportional to the concentration of [TG(GA)3]n. For example, the [TG(GA)3]2 concentration increased from 1 × 10−7 M to 2 × 10−7 M, and the corresponding fluorescence intensity of [TG(GA)3]2/ThT also increased and doubled. Furthermore, we compared the fluorescence intensity of [TG(GA)3]n/ThT with different concentrations and different numbers of TG(GA)3 motifs (n). It can be seen from Fig. S1B† that the fluorescence intensities of [TG(GA)3]n/ThT are about the same when C × n (where C is the concentration of [TG(GA)3]n, and n is the number of TG(GA)3 motifs) is equal (such as, 1 × 4 = 2 × 2, 1 × 6 = 2 × 3 = 3 × 2). These results confirm that TG(GA)3 is the basic unit for binding ThT in the parallel [TG(GA)3]n-homoduplex. On the other hand, we measured the viscosity of the DNA solutions with different sequences and different concentrations (0.5 × 10−7 mol L−1, 0.7 × 10−7 mol L−1 and 1 × 10−7 mol L−1). The experimental results showed that the viscosity of the DNA solutions was almost the same as the viscosity of the solvent (H2O), and the viscosity of the DNA solutions did not change with changing DNA sequences and concentrations. So, the effect of solution viscosity on fluorescence could be ruled out in this system.
The G-quadruplex (G4) from one G-rich strand (5′-AGG GTT AGG GTT AGG GTT AGG GTT AGG G-3′) was reported to possess the highest enhancement factor for ThT fluorescence.5 The parallel dsDNA from (GA)10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 and TG(GA)10
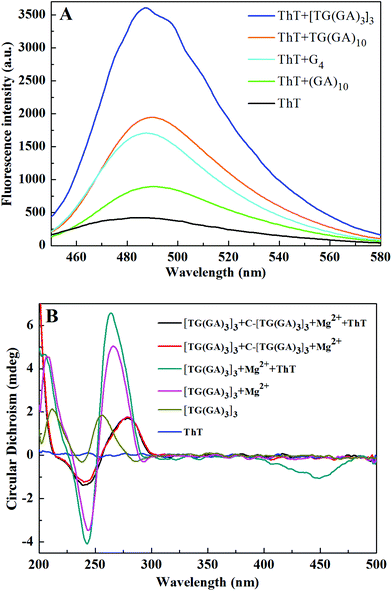
31 and TG(GA)10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 is reported to enhance the fluorescence of ThT. For comparison, under the same experimental conditions, we measured the fluorescence of ThT in the presence of [TG(GA)3]3, G4, (GA)10 and TG(GA)10. As indicated in Fig. 3A, the fluorescence intensity of [TG(GA)3]3/ThT is higher than those of (GA)10/ThT and TG(GA)10/ThT, and is 2-fold higher than that of G4/ThT. Thus [TG(GA)3]3/ThT was selected for the following experiments.
32 is reported to enhance the fluorescence of ThT. For comparison, under the same experimental conditions, we measured the fluorescence of ThT in the presence of [TG(GA)3]3, G4, (GA)10 and TG(GA)10. As indicated in Fig. 3A, the fluorescence intensity of [TG(GA)3]3/ThT is higher than those of (GA)10/ThT and TG(GA)10/ThT, and is 2-fold higher than that of G4/ThT. Thus [TG(GA)3]3/ThT was selected for the following experiments.
Structure characterization of the parallel [TG(GA)3]3-homoduplex and [TG(GA)3]3-dsDNA/ThT
CD spectroscopy is a common technique for studying the DNA structure.33 We also recorded the CD spectra of [TG(GA)3]3 under different conditions. As indicated in Fig. 3B, the addition of Mg2+ induces one positive peak at 266 nm and one negative peak at 244 nm in the CD spectrum, which is characteristic of a parallel DNA duplex,32,34 suggesting that a parallel DNA duplex forms from [TG(GA)3]3 in the presence of Mg2+. Upon addition of ThT into the [TG(GA)3]3–Mg2+ system, the characteristic CD peaks of parallel dsDNA increase slightly, and at the same time, a new negative CD peak appears at ca. 450 nm, which suggests that ThT interacts with the parallel DNA duplex through an intercalation mode of binding.5,32 For comparison, the CD spectrum of a mixture of [TG(GA)3]3 and its complementary DNA (denoted by C-[TG(GA)3]3) was also recorded, and the CD spectrum displayed one positive peak at 280 nm and one negative peak at 240 nm, which is characteristic of an antiparallel DNA duplex.33 Addition of ThT into antiparallel dsDNA (C-[TG(GA)3]3 + [TG(GA)3]3) did not induce any change in the CD spectrum. These results demonstrated that ThT can interact only with the parallel [TG(GA)3]3 homoduplex, but cannot interact with the antiparallel DNA duplex. Furthermore, to verify that [TG(GA)3]3 DNA can self-associate into a stable homoduplex structure, polyacrylamide gel electrophoresis experiment was performed to characterize the formation of a parallel [TG(GA)3]3 homoduplex. In Fig. S2,† lane 1 shows the band of C-[TG(GA)3]3, and lane 2 shows the band of the antiparallel duplex formed from C-[TG(GA)3]3 and [TG(GA)3]3, which was used as a control for ps[TG(GA)3]3. We could see an allelic band (25 bp) in lane 3, which demonstrated that [TG(GA)3]3 had formed homoduplexes as anticipated.Fluorescence stability of the parallel [TG(GA)3]3-homoduplex/ThT indicator
Signal stability is an especially important property for fluorescent probes or indicators, which makes fluorescence detection or biosensing more reliable and accurate. It was reported that Mg2+ was a stabilizer for GA-containing parallel double stranded DNA.32,34 We systematically explored the effect of Mg2+ concentration on the fluorescence intensity of [TG(GA)3]3-homoduplex/ThT. The experimental result (Fig. S3†) showed that 20 mM Mg2+ could induce a stable and strong fluorescence intensity. So, we added 20 mM Mg2+ into the [TG(GA)3]n/ThT system. We compared the fluorescence intensity of the parallel [TG(GA)3]3/ThT system in different media, including solutions containing different concentrations of Tris–HCl, K+, Na+ and NH4+. As the control, the fluorescence intensity of the G4/ThT system was also measured under the same conditions. As shown in Fig. 4A, when the Na+ concentration increases from 0 to 200 mM, the fluorescence intensity of the parallel [TG(GA)3]3/ThT system remains almost unchanged, whereas the fluorescence intensity of the G4/ThT system rapidly decreases to about 20%. Almost similarly, the fluorescence intensity of the [TG(GA)3]3/ThT system did not significantly change by increasing the concentration of K+ (Fig. 4B), NH4+ (Fig. 4C) or Tris–HCl (Fig. 4D) in the 0–200 mM range; by contrast, the concentration change of K+, NH4+ or Tris–HCl in the range of 0–200 mM would induce an obvious change in the fluorescence intensity of the G4/ThT system. These results demonstrated that parallel [TG(GA)3]3/ThT is a stable fluorescent indicator, whereas G4/ThT fluorescence is rather unstable.In order to understand why the fluorescence stability of these systems is different, we recorded the CD spectra of the parallel [TG(GA)3]3/ThT system or G4/ThT system in different media. As shown in Fig. 5A, C, E and G, the CD spectra of parallel [TG(GA)3]3/ThT almost remain unchanged when the concentration of Na+, K+, NH4+ or Tris–HCl increases from 0 to 200 mM. That is to say that the conformation of parallel [TG(GA)3]3/ThT is stable in different media. The hydrogen bonds between G–G and A–A base pairs make the [TG(GA)3]3 strand self-associate into a stable parallel DNA duplex structure.32 The parallel [TG(GA)3]3-duplex has only one conformation, and the conformation of the parallel [TG(GA)3]3-duplex does not change. Therefore, the stable structure of parallel [TG(GA)3]3/ThT results in the stable fluorescence intensity in different media. However, it is well known that G4 can fold into parallel, antiparallel, and mixed parallel/antiparallel conformations. The G4 conformation is sensitive to the ion concentrations, and the change of ion concentrations can induce the topological transformation of G4.5 So, the CD spectra of G4/ThT changed with the change in Na+, K+, NH4+, or Tris–HCl concentrations in the range of 0–200 mM (Fig. 5B, D, F and H), resulting in the unstable fluorescence intensity of G4/ThT in different media.
Isothermal exponential amplification reaction-based biosensing system using parallel [TG(GA)3]3-homoduplex/ThT as the fluorescent indicator
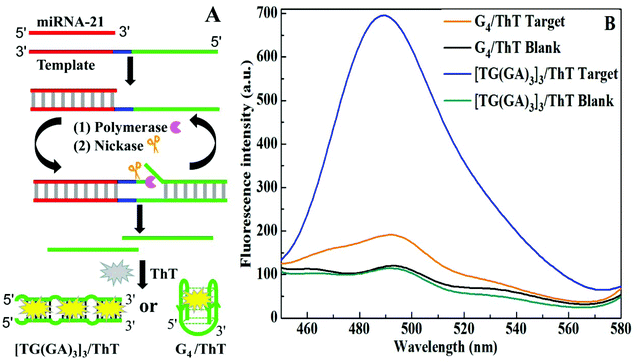
An isothermal exponential amplification reaction (EXPAR) can cause a molecular recognition event to generate a large number of short DNAs.35 In order to demonstrate the utility of this stable and intense fluorescent indicator, we applied parallel [TG(GA)3]3-homoduplex/ThT for constructing the EXPAR strategy for microRNA detection. As depicted in Fig. 6A, the template contains three domains: the recognition region for target microRNA (red), the nicking site of nicking endonuclease (blue) and the signal region for generating [TG(GA)3]3 (green). In order to prevent the non-specific extension of the template, the 3′ terminus of the template was modified with a phosphate group. The hybridization of the template and target microRNA triggered the EXPAR process. The microRNA/template partial duplex was extended in the presence of DNA polymerases and dNTPs, generating a whole dsDNA with one nicking site for nicking endonuclease. The cleavage of the nicking site would release the signal DNA ([TG(GA)3]3), meanwhile the cleavage reaction would initiate a new cycle of extension–cleavage–release. Through many cycles, a large number of signal DNAs could be produced. The [TG(GA)3]3 self-assembled into a stable parallel DNA duplex, and then bound with ThT to generate fluorescent signals. For comparison, we also used the EXPAR system to produce G4/ThT. As shown in Fig. 6B, the fluorescence intensity of the [TG(GA)3]3/ThT system is about 3.6-fold higher than that of the G4/ThT system. Additionally, under the optimal experimental conditions of the EXPAR-[TG(GA)3]3/ThT system (Fig. S4 and S5†), we investigated the luminescence response of the system to different concentrations of miRNA-21 ranging from 0 to 5 nM. The result (Fig. S6A†) indicated that as the miRNA-21 concentration was increased, the fluorescence intensity increased accordingly, and a linear relationship (F = 117.28C + 101.41, R2 = 0.9953) between the fluorescence intensity at 490 nm and the miRNA-21 concentration in the 0.075–5 nM range was observed as shown in Fig. S6B.† The detection limit (3σ) was estimated to be 0.05 nM, which was lower than that obtained with the EXPAR-G4/ThT system (Fig. S7†). Therefore, the sensitivity of the [TG(GA)3]3/ThT probe was higher than that of the G4/ThT probe.Conclusions
In this work, we suggested a new label-free fluorescent indicator, parallel [TG(GA)3]n-homoduplexes/ThT. Compared to the traditional G-quadruplex/ThT probe, the parallel [TG(GA)3]n-homoduplexes/ThT probe exhibited more stable and stronger fluorescence. We used the parallel [TG(GA)3]3-homoduplexes/ThT as the fluorescent indicator to develop a simple and sensitive EXPAR-based biosensing platform for label-free detection of microRNA. This work demonstrates that parallel [TG(GA)3]n-homoduplexes/ThT is an intense and stable fluorescent indicator for label-free biosensing. So, this work opens a new paradigm to develop some simple and sensitive methods for label-free biosensing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21775099 and 21974082).References
- A. I. Sulatskaya, I. M. Kuznetsova and K. K. Turoverov, Interaction of thioflavin T with amyloid fibrils: fluorescence quantum yield of bound dye, J. Phys. Chem. B, 2012, 116, 2538–2544 CrossRef CAS.
- N. Amdursky, Y. Erez and D. Huppert, Molecular rotors: what lies behind the high sensitivity of the Thioflavin-T fluorescent marker, Acc. Chem. Res., 2012, 45, 1548–1557 CrossRef CAS.
- S. M. Saeed and G. Fine, Thioflavin-T for amyloid detection, Am. J. Clin. Pathol., 1967, 47, 588–593 CrossRef CAS.
- B. Bulic, M. Pickhardt, B. Schmidt, E. M. Mandelkow, H. Waldmann and E. Mandelkow, Development of tau aggregation inhibitors for alzheimer's disease, Angew. Chem., Int. Ed., 2009, 48, 1740–1752 CrossRef CAS.
- J. Mohanty, N. Barooah, V. Dhamodharan, S. Harikrishna, P. I. Pradeepkumar and A. C. Bhasikuttan, Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA, J. Am. Chem. Soc., 2012, 135, 367–376 CrossRef.
- A. R. de la Faverie, A. Guédin, A. Bedrat, L. A. Yatsunyke and J.-L. Mergny, Thioflavin T as a fluorescence light-up probe for G4 formation, Nucleic Acids Res., 2014, 42, e65 CrossRef.
- F. Y. Khusbu, X. Zhou, H. Chen, C. Ma and K. Wang, Thioflavin T as a fluorescence probe for biosensing applications, Trends Anal. Chem., 2018, 109, 1–18 CrossRef.
- X. Tan, Y. Wang, B. A. Armitage and M. P. Bruchez, Label-free molecular beacons for bio-molecular detection, Anal. Chem., 2012, 84, 7664–7669 CrossRef.
- H. Zhao, J. Dong, F. Zhou and B. Li, G-quadruplex-based homogenous fluorescence platform for ultrasensitive DNA detection through isothermal cycling and cascade signal amplification, Microchim. Acta, 2015, 182, 2495–2502 CrossRef CAS.
- Z. Jin, D. Geißler, X. Qiu, K. D. Wegner and N. Hildebrandt, A rapid, amplification-free, and sensitive diagnostic assay for single-step multiplexed fluorescence detection of microRNA, Angew. Chem., Int. Ed., 2015, 54, 10024–10029 CrossRef CAS.
- H. Fujita, Y. Kataoka, S. Tobita, M. Kuwahara and N. Sugimoto, A novel one-tube-one-step real-time methodology for rapid transcriptomic biomarker detection: signal amplification by ternary initiation complexes, Anal. Chem., 2016, 88, 7137–7144 CrossRef CAS.
- D. Bai, D. Ji, J. Shang, Y. Hu, J. Gao, Z. Lin and Z. Li, A rapid biosensor for highly sensitive protein detection based on G-quadruplex-Thioflavin T complex and terminal protection of small molecule-linked DNA, Sens. Actuators, B, 2017, 252, 1146–1152 CrossRef CAS.
- X. Liu, X. Hua, Q. Fan, J. Chao, S. Su, Y. Q. Huang, L. Wang and W. Huang, Thioflavin T as an efficient G-quadruplex inducer for the highly sensitive detection of thrombin using a new foster resonance energy transfer system, ACS Appl. Mater. Interfaces, 2015, 7, 16458–16465 CrossRef CAS.
- Z. Wang, J. Zhao and Z. Dai, A label-free fluorescent adenosine triphosphate biosensor via overhanging aptamer-triggered enzyme protection and target recycling amplification, Analyst, 2016, 141, 4006–4009 RSC.
- H. Wang, P. Peng, S. Liu and T. Li, Thioflavin T behaves as an efficient fluorescent ligand for label-free ATP aptasensor, Anal. Bioanal. Chem., 2016, 408, 7927–7934 CrossRef CAS.
- L. Ma, Z. Zhang, M. Wang, L. Lu, H. J. Zhong and C. H. Leung, Recent developments in G-quadruplex probes, Chem. Biol., 2015, 22, 812–828 CrossRef.
- D. Miyoshi, H. Karimata and N. Sugimoto, Hydration regulates thermodynamics of G-quadruplex formation under molecular crowding conditions, J. Am. Chem. Soc., 2006, 128, 7957–7963 CrossRef CAS.
- Y. Xue, Z. Y. Kan, Q. Wang, Y. Yao, J. Liu, Y. H. Hao and Z. Tan, Human telomeric DNA forms parallel-stranded intramolecular G-quadruplex in K+ solution under molecular crowding condition, J. Am. Chem. Soc., 2006, 129, 11185–11194 CrossRef.
- Y. Wang and D. J. Patel, Solution structure of the human telomeric repeat d [AG3(T2AG3)3] G-tetraplex, Structure, 1993, 1, 263–282 CrossRef CAS.
- D. Monchaud, P. Yang, L. Lacroix, M. P. Teulade-Fichou and J. L. Mergny, A metal-mediated conformational switch controls G-quadruplex binding affinity, Angew. Chem., Int. Ed., 2010, 47, 4858–4861 CrossRef.
- K. W. Lim, S. Amrane, S. Bouaziz, W. Xu, Y. Mu, D. J. Patel and A. T. Phan, Structure of the human telomere in K+ solution: a stable basket-type G-quadruplex with only two G-tetrad layers, J. Am. Chem. Soc., 2009, 131, 4301–4309 CrossRef CAS.
- A. T. Phan and D. J. Patel, Two-repeat human telomeric d(TAGGGTTAGGGT) sequence forms interconverting parallel and antiparallel G-quadruplexes in solution: distinct topologies, thermodynamic properties, and folding/unfolding kinetics, J. Am. Chem. Soc., 2003, 125, 15021–15027 CrossRef CAS.
- E. M. Rezler, J. Seenisamy, S. Bashyam, M. Y. Kim, E. White, W. D. Wilson and L. H. Hurley, Telomestatin and diseleno sapphyrin bind selectively to two different forms of the human telomeric G-quadruplex structure, J. Am. Chem. Soc., 2005, 127, 9439–9447 CrossRef CAS.
- V. Dhamodharan, S. Harikrishna, C. Jagadeeswaran, K. Halder and P. I. Pradeepkumar, Selective G-quadruplex DNA stabilizing agents based on bisquinolinium and bispyridinium derivatives of 1, 8-naphthyridine, J. Org. Chem., 2012, 77, 229–242 CrossRef CAS.
- S. D. Verma, N. Pal, M. K. Singh, H. Shweta, M. F. Khan and S. Sen, Understanding ligand interaction with different structures of G-quadruplex DNA: evidence of kinetically controlled ligand binding and binding-mode assisted quadruplex structure alteration, Anal. Chem., 2012, 84, 7218–7226 CrossRef CAS.
- J. H. Van De Sande, N. B. Bamsing, M. W. Germann, W. Blhorst, B. W. Kalisch, B. V. Kitzing, R. T. Pon, R. C. Clegg and T. M. Jovin, Parallel stranded DNA, Science, 1988, 241, 551–557 CrossRef CAS.
- X. Ming, P. Ding, P. Leonard, S. Budow and F. Seela, Parallel- stranded DNA: enhancing duplex stability by the ‘G-clamp’ and a pyrrolo-dC derivative, Org. Biomol. Chem., 2012, 10, 1861–1869 RSC.
- I. Sinha, C. F. Guerra and J. Müller, A highly stabilizing silver(I)-mediated base pair in parallel-stranded DNA, Angew. Chem., Int. Ed., 2015, 54, 1–5 CrossRef.
- M. Y. Ye, R.-T. Zhu, X. Li, X.-S. Zhou, Z. Z. Yin, Q. Li and Y. Shao, Adaptively recognizing parallel-stranded duplex structure for fluorescent DNA polarity analysis, Anal. Chem., 2017, 89, 8604–8608 CrossRef CAS.
- M. Szabat and R. Kierzek, Parallel-stranded DNA and RNA duplexes-structural features and potential applications, FEBS J., 2017, 284, 3986–3998 CrossRef CAS.
- S. Liu, P. Peng, H. Wang, L. Shi and T. Li, Thioflavin T binds dimeric parallel-stranded GA-containing non-G-quadruplex DNAs: a general approach to lighting up double-stranded scaffolds, Nucleic Acids Res., 2017, 45, 12080–12089 CrossRef CAS.
- J. Zhu, Z. Yan, W. Zhou, C. Liu, J. Wang and E. Wang, Lighting up the Thioflavin T by parallel-stranded TG(GA)n DNA homoduplexes, ACS Sens., 2018, 3, 1118–1125 CrossRef CAS.
- J. Kypr, I. Kejnovska, D. Renciuk and M. Vorlickova, Circular dichroism and conformational polymorphism of DNA, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS.
- K. Rippe, V. Fritsch, E. Westhof and T. M. Jovin, Alternating d(GA) sequences form a parallel-stranded DNA homoduplex, EMBO J., 1992, 11, 3777–3786 CrossRef CAS.
- S. Reid, X. C. Le and H. Zhang, Exponential isothermal amplification of nucleic acids and assays for proteins, cells, small molecules, and enzyme activitieseAn EXPAR example, Angew. Chem., Int. Ed., 2018, 90, 11856–11866 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9an01856e |
| This journal is © The Royal Society of Chemistry 2020 |