Rethinking and new perspectives on cardiotoxicity of traditional Chinese medicine
Lizhen
Qiu†
ab,
Wei
Zhou†
 b,
Hongling
Tan
b,
Xianglin
Tang
b,
Yuguang
Wang
b,
Zengchun
Ma
b and
Yue
Gao
*ab
b,
Hongling
Tan
b,
Xianglin
Tang
b,
Yuguang
Wang
b,
Zengchun
Ma
b and
Yue
Gao
*ab
aTianjin University of Traditional Chinese Medicine, Tianjin 301617, PR China. E-mail: gaoyue@bmi.ac.cn
bDepartment of Pharmaceutical Sciences, Beijing Institute of Radiation Medicine, Beijing, 100850, China
First published on 16th November 2018
Abstract
Traditional Chinese Medicine (TCM) has been commonly used in clinical practice for thousands of years and has made enormous contributions to public health in China. However, the adverse effects on the cardiac system or TCM-induced cardiovascular diseases have emerged frequently in recent years, resulting in growing attention to the safety of TCM. Generally, TCM with adverse cardiac effects has typical therapeutic or toxic effects, which are based on specific material basis for efficacy/toxicity, specific clinical symptoms and toxic mechanisms. However, improper strategies adopted for research on the cardiotoxicity of TCM simply follow the basic principles of conventional toxicology and cause exaggerative or incorrect interpretations in the toxicity of TCM. In this review, we aim to present the classification and possible toxic mechanisms for TCM with cardiotoxicity based on the material basis for toxicity to rethink the existing problems in toxicity studies for TCM and provide new perspectives for research on the potential cardiotoxicity of TCM. We hope that this study can offer important theoretical support and scientific advice for the toxicity study and clinical rational use of TCM having cardiotoxicity.
1. Introduction
TCM, as a traditional treasure, has been clinically practiced in China for centuries due to the excellent reputation for preventing and healing diseases, and it has also made great contributions to human health. In recent years, along with its industrialization and internationalization, TCM has played an indispensable role in maintaining the health of people in China and several Asian regions.1,2 Besides, many people are more inclined to choose TCM primarily as a complementary health approach or dietary supplements in Europe and America.3 However, there is also clear rise in the occurrence of adverse events or drug-induced side effects, all of which largely result from insufficient understanding or partial knowledge of the toxicity and pharmacology of TCM during clinical treatment.4 Many reports have demonstrated that TCM might cause liver damage, kidney injury and cardiotoxicity.5,6 Particularly, TCM-induced cardiotoxicity has attracted more attention owing to its high mortality rate. In addition, many TCM, which have been used in clinical practice for years have been withdrawn from the market or some restrictions have been imposed on their use because of their potential cardiotoxicity, which was not taken into much consideration in preclinical studies.7 Thus, cardiac toxicity caused by TCM is not only a major concern in clinical treatment but also a matter needing more attention during the drug development process. However, some previous studies report toxicity testing on TCM independent of the guidance of traditional Chinese medicine (TCM) theories and indicate improper conclusions on the toxicity of TCM having cardiotoxicity. Herein, we focused on the classification of TCM having cardiotoxicity and advances in their possible toxic mechanisms, which are mainly based on the material basis for toxicity and clinical symptoms. Lastly, we presented existing problems in toxicology studies on cardiotoxic TCM and put forward some new thoughts and strategies towards toxicity research on TCM according to the guidance of TCM theories, aiming to provide scientific advice for TCM-induced cardiac damage and clinical rational use of cardiotoxic TCM.2. The material basis for the cardiotoxicity of TCM
Cardiotoxic TCM has a wide range of pharmacological activities or therapeutic effects, all of which promote the widespread use in clinical treatments. For example, most aconitum plants play an important role in anti-inflammatory, analgesic, anti-tumor and anti-arrhythmia treatments8 even though there are diester diterpenoid alkaloids (the main toxic material basis, for instance, aconitine) existing in most Aconitum Ranunculaceae. Tripterygium wilfordii Hook f., also belonging to cardiotoxic TCM with clear therapeutic effects, is widely used in the clinical treatment of rheumatoid arthritis and systemic lupus erythematosus because of remarkable anti-inflammatory and immunosuppressive effects produced by its major active ingredient, triptolide.9 Toxic glycosides including cardiac glycosides, steroidal saponins, triterpene saponins, and cyanogenic glycosides are a class of compounds with remarkable biological effects on cardiac function and are clinically used for the treatment of chronic heart failure.10,11 Toad venom (“Chansu” ( )) is a rare kind of TCM that has complex chemical components including bufadienolides, indole alkaloids, and cholesterol metabolites; it has been widely used in clinical practice and displays various pharmaceutical effects such as cardiotonic, anti-tumor, analgesic, and local anesthetic effects.12
)) is a rare kind of TCM that has complex chemical components including bufadienolides, indole alkaloids, and cholesterol metabolites; it has been widely used in clinical practice and displays various pharmaceutical effects such as cardiotonic, anti-tumor, analgesic, and local anesthetic effects.12
However, poor understanding of the undesirable side of the main pharmacological components easily attracts minimal attention for their potential cardiotoxicity. These main biological/pharmacological components accumulate in the body and always act as the main material basis for the adverse cardiac effects of TCM with toxicity.13 Thus, these TCM have a narrow dose- or time-window and always cause clear adverse cardiac effects after long-term administration or under different physical conditions. Eventually, TCM-induced adverse cardiac effects have been recognized as one of the common reasons for their restricted clinical use or preclinical-trial failures, even in withdrawal. Meanwhile, systematic characterization and understanding of these TCM are hindered by multiple effects and multiple mechanisms produced by complex multi-components.14 Therefore, there is a need to develop a simple and precise classification for numerous cardiotoxic TCM, which will not only contribute to the comprehensive knowledge of TCM, but also promote the research and development of new TCM with low toxicity and high efficiency.
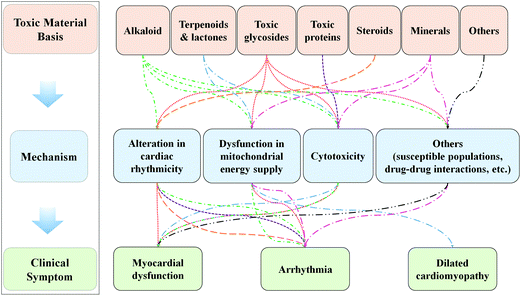
In this review, we presented a classification for cardiotoxic TCM, which mainly consisted of three categories: herbs, animal-based medicines and mineral medicines (as shown in Table 1),15 among which botanical drugs account for a significant proportion. Then, the three main types of TCM can also be further classified according to their main toxic components.16 For example, herbs can be classified into alkaloids, toxic glycosides, terpenes and lactones, toxic proteins and others. As a very special part of TCM, animal-based medicines (including Scorpio, Scolopendra, Bungarus Parvus, Mylabris and Bufonis Venenum) can be divided into two types, i.e., toxic proteins and cardiac glycosides by referring to their major toxic ingredients. Mineral medicines are always regarded as types of therapeutic substances with extreme toxicity and can be classified as arsenical, mercury, and lead, copper and iron according to their main mineral elements. This classification is based on the material basis for the toxicity of TCM and can help us study their potential cardiotoxicity and toxic mechanisms involved.
| Classification | Main toxic components | Names |
|---|---|---|
| Herb medicines | Alkaloid | Aconiti Radix; Aconiti Kusnezoffii Radix; Aconiti Lateralis Radix Praeparata; Daturae Flos; Ephedrae Herba; Belladonnae Herba; Strychni Semen; Papaveris Pericarpium; Tripterygium wilfordii; Tripterygium hypoglaucum(level.) Hutch.; Macleaya cordata; Gelsemium elegans; Xueshangyizhihao; Veratrum nigrumL.; Datura stramonium Linn.; Cicuta virosa L.; Solanum nigrum L. |
| Toxic glycosides | Apocyni Veneti Folium; Cynanchi Atrati Radix Et Rhizoma; Akebiae Caulis; Polygonati Odorati Rhizoma; Periplocae Cortex; Acanthopanacis Cortex; Arisaematis Rhizoma; Pulsatillae Radix; Ophiopogonis Radix; Allii Macrostemonis Bulbus; Paridis Rhizoma; Lilii Bulbus; Tribuli Fructus; Ginseng Radix Et Rhizoma; Notoginseng Radix Et Rhizoma; Bupleuri Radix; Platycodonis Radix; Glycyrrhizae Radix Et Rhizoma; Phytolaccae Radix; Anemarrhenae Rhizoma; Armeniacae Semen Amarum; Persicae Semen; Pruni Semen; Dioscorea bulbifera L. Rhizoma; Gleditsia sinensis Lam. Fructus; Digitalis; Semen Strophanthi Divaricati; Nerium indicum Mill.; Urginea maritima; Rohdea Roth; Convallaria majalis Linn.; Niuxinqie; Manihot esculenta Crantz; Antiaris toxicaria Lesch.; Periploca sepium Bunge.; Solanum lyratum; Eriobotryae Semen | |
| Terpenoids and lactones | Mylabris; Asari Radix Et Rhizoma; Bruceae Fructus; Toosendan Fructus; Phytolaccae Radix; Euphorbiae Semen; Dioscorea bulbifera L. Rhizoma; Meliae Fructus; Tripterygium wilfordii; Lytta; Lllicium henryi Diels | |
| Toxic proteins | Lablab Semen Album; Xanthii Fructus; Ricini Semen; Bruceae Fructus; Trichosanthis Radix; Crotonis Fructus; Dioscorea bulbifera L. Rhizoma; Meliae Frutus; Abrus precatorius L.; Lllicium henryi Diels | |
| Others | Ginkgo Semen; Bruceae Fructus; Kansui Radix; Polygoni Multiflori Radix; Chebulae Fructus; Polygoni Cuspidati Rhizoma Et Radix; Cassia occidentalis L. | |
| Animal-based medicines | Toxic proteins | Hirudo; Scolopendra; Agkistrodon; Bungarus Parvus; Scorpio; Mylabris |
| Steroids | Bufonis Venenum | |
| Mineral medicines | Arsines | Realgar(As2S2); Arsenolite (FeAsS); Arsenic(As2O3); Arsenblende(As2S3) |
| Mercury | Cinnabaris(HgS); Hydrargyri Oxydum Rubrum(HgO); Calomelas(Hg2Cl2); Hydrargyrum oxydatum crudum(HgO); Mercury sulphide(HgS) | |
| Lead | Red lead (Pb3O4); Lithargyrum (Pb3O4); Lead powder; Lead frost | |
| Iron and copper | Pyritum(FeS2); Haematitum (Fe2O3); Limonitum((FeO(OH)); Magnetitum (Fe3O4); Chalcanthitum (CuSO4·5H2O); Zingar (2Cu2(OH)3Cl); Azurite (2CuCO3·Cu(OH)2); Copper carbonate basic (Cu2(OH)2CO3) |
3. TCM-induced cardiotoxicity and potential underlying mechanisms
Drug-induced cardiotoxicity is a branch of adverse responses that are undesired effects on cardiac tissues and are directly or indirectly caused by drugs during clinical treatments or preclinical tests17 such as arrhythmia, myocardial ischemia or dysfunction, and dilated cardiomyopathy. Luckily, drug- or TCM-induced cardiotoxicity can be often characterized by special clinical symptoms: chest pain, shortness of breath, palpitation, cyanosis of the extremities and lips, pale complexion, cold limbs, and hypotension, all of which are always known in TCM theory as palpitation (“Zheng Chong” ( )), thoracic obstruction (“Bi” (
)), thoracic obstruction (“Bi” ( )), and chest bind syndrome (“Jie Xiong” (
)), and chest bind syndrome (“Jie Xiong” ( )). These clinical symptoms provide a basis for the research on potential toxic mechanisms underlying TCM-induced cardiotoxicity.
)). These clinical symptoms provide a basis for the research on potential toxic mechanisms underlying TCM-induced cardiotoxicity.
Nowadays, an increasing number of studies have demonstrated that TCM-induced alteration in the rhythmicity of myocytes or autorhythmic cells is the principal reason for TCM-caused arrhythmia, which is also one of the most common and lethal clinical symptoms caused by cardiotoxic TCM. Besides, as the primary source of energy supply, mitochondria are of great importance for the maintenance of myocardia homeostasis but are also the toxic targets of some cardiotoxic TCM.18 Moreover, some cardiotoxic TCM can directly cause cytotoxicity and other toxic effects on myocytes. Hence, alterations in cardiac auto-rhythmicity, dysfunction in mitochondrial energy supply, and cytotoxicity induced by the material basis for TCM might play critical roles in TCM-induced clinical symptoms (Fig. 1).
3.1.Alteration in cardiac rhythmicity
Studies have demonstrated that many TCM with cardiac toxicity can directly impose adverse effects on the ionic channels of myocytes or autorhythmic cells, change the concentrations of intracellular/extracellular ions and perturb the rhythmic activity of cardiac cells.19 For example, some alkaloid and cardiac glycosides can alter the intracellular concentrations of ions (Na+, Ca2+ or Mg2+, K+) and promote myocyte depolarization by inhibiting the activity of Na+ channels, sodium-calcium exchanger or related enzymes (Ca2+-ATP enzyme, Na+-K+-ATP enzyme and Ca2+-Mg2+-ATP enzyme);20–22 also, as shown in our previous work and some articles, alkaloids have been reported to disturb intracellular calcium homeostasis by enhancing the expression of calmodulin,23,24 all of which eventually lead to myocyte depolarization and various types of ventricular arrhythmia. Besides, it is reported that some herbal medicines can trigger arrhythmia by exerting effects on the cardiac vagus nerve and postganglionic acetylcholine release to reduce the auto-rhythmicity and conductivity of the sinoatrial node or by directly inhibiting the atrioventricular pacemaker or the cardiac conduction system.25 Cinobufagin (CBG), a major bioactive ingredient of ChanSu, has been widely used in the treatment of coronary heart diseases and was also reported to trigger abnormal action potential in myocytes through dose-dependent L-type calcium current blocking in rat ventricles.263.2 Dysfunction in the mitochondrial energy supply
Generally, oxidative stress-mediated dysfunction in the mitochondrial energy supply has been shown to be related to TCM-induced cardiotoxicity. Metabonomic studies have indicated that TCM-containing alkaloids (Fuzi (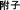 ) aqueous extract or aconitine) can destroy the balance between the oxidation and antioxidation systems or directly suppress the formation of the tricarboxylic acid (TCA) cycle and interact with the electron transport chain, following ROS generation, lower activity of respiratory chain-related enzymes (Cytochrome C and NADHD) and clear repression of mitochondrial membrane potential (MMP) and ATP content in rat myocytes.27,28 Recent research has suggested that triptolide promotes the accumulation of ROS and suppresses downstream antioxidants, which is associated with the mitochondria-mediated apoptotic signaling pathway.29 Moreover, toxic herbs containing terpenoid compounds can easily penetrate the mitochondrial membrane and produce excessive free radicals, which can inhibit the activity of enzymes in respiratory chain complexes and the levels of oxidative phosphorylation in the mitochondria, leading to the reduction of ATP content in myocytes.30 Arsenic trioxide was also reported to cause ROS-mediated ultrastructural changes in mitochondria such as swelling, cristae disruption, and vacuolization, all of which can inhibit ATP production in cardiac tissues.31
) aqueous extract or aconitine) can destroy the balance between the oxidation and antioxidation systems or directly suppress the formation of the tricarboxylic acid (TCA) cycle and interact with the electron transport chain, following ROS generation, lower activity of respiratory chain-related enzymes (Cytochrome C and NADHD) and clear repression of mitochondrial membrane potential (MMP) and ATP content in rat myocytes.27,28 Recent research has suggested that triptolide promotes the accumulation of ROS and suppresses downstream antioxidants, which is associated with the mitochondria-mediated apoptotic signaling pathway.29 Moreover, toxic herbs containing terpenoid compounds can easily penetrate the mitochondrial membrane and produce excessive free radicals, which can inhibit the activity of enzymes in respiratory chain complexes and the levels of oxidative phosphorylation in the mitochondria, leading to the reduction of ATP content in myocytes.30 Arsenic trioxide was also reported to cause ROS-mediated ultrastructural changes in mitochondria such as swelling, cristae disruption, and vacuolization, all of which can inhibit ATP production in cardiac tissues.31
3.3 Cytotoxicity
As documented in recent research, prolonged administration of some Chinese herbs even at non-acute toxic doses might cause inflammatory responses, myocardial tissue dissolution and necrosis. Toxic TCM-induced direct injury on cardiac cells can promote the release of many metabolites and ions (such as K+) from the cytoplasm and cause local inflammation and high local potassium concentrations, which can in turn trigger tachyarrhythmia.32 Some animal experiments have shown that high doses of alkaloids can induce significant pathological ultrastructural changes, such as disordered arrangement of myofibrils, shortening of pseudopodia and cytoplasmic vacuoles, degranulation, and even cell necrosis, all of which have been associated with abnormal dephosphorylation of specific sites of Cx43 protein.33 Besides, our previous study showed that Panax notoginseng saponins (PNS), one of the toxic glycosides, dose-dependently inhibited the viability of H9C2 cells, which may be related to the upregulated expressions of CYP4A3 and CYP4F4.34 In another study, the authors found that another toxic glycoside, ophiopogon saponins D′, can induce mitochondrion-dependent apoptosis in rat myocytes by increasing intracellular ROS generation while decreasing MMP,35 all of which can be important reasons for TCM-induced myocardial fibrosis and chronic heart failure after long-term use. Furthermore, some toxic proteins are not documented to induce visible heart damage, but evaluated serum aspartate aminotransferase (AST) indicates potential cytotoxicity on the cardiac system.36,373.4 Others (drug–drug interactions, susceptible populations, vascular toxicity, etc.)
Given that TCM is characterized by multiple components, multiple targets, and multiple pathways, in addition to the cardiotoxic mechanisms mentioned above, we should also pay more attention to other corresponding toxic mechanisms underlying TCM-induced cardiotoxicity. Some Chinese medicinal herbs, such as aconitum alkaloids and digitalis (one of the toxic glycosides), can interact with certain chemical drugs and affect their metabolism and blood concentration, subsequently leading to drug accumulation and drug-induced myocardial injury.13,38,39 Besides, some special populations, including elders and patients with heart failure, electrolyte disorder, or myocardial ischemia, are more susceptible to different types of alkaloid or cardiac glycoside-induced arrhythmia.40 Moreover, previous studies have shown that some toxic TCMs exhibit clear vascular toxic effects by enhancing the vasoconstriction of coronary arteries and promoting platelet aggregation and thrombus formation. For example, lead increased vascular contractile responses to norepinephrine and exacerbated myocardial load and ischemia.414. New thinking and perspectives on the studies of TCM-induced cardiotoxicity
De facto, TCM has existed in the market for a long time and is at the stage of post-marketing safety monitoring, but there is a serious dearth of safety data for TCM toxicity. Hence, toxicity risk assessment for TCM is an important and intriguing field in the Chinese Materia Medica. Recently, facing an increasing need for safe evaluation of TCM, more and more studies on the cardiotoxicity of TCM are overly launched using modern toxicology approaches and indeed exhibit substantial toxicity data, which unfortunately always causes a toxicity paradox or contradictory conclusions for these traditional medicines. Parts of major contributors to TCM-induced cardiotoxicity have been well documented in previous studies including the quality controls associated with GLPs and potential contaminants in TCM and will not be discussed in this article. Therefore, the three critical problems in cardiotoxicity assessment for TCM and new perspectives discussed below may provide scientific guidance for future research.4.1 Efficacy or toxicity? More association with physical conditions
About 2000 years ago, the theory of “You Gu Wu Yun” (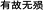 ) was put forward by “Huangdi Neijing” (
) was put forward by “Huangdi Neijing” ( ), the bible of TCM; “Wu Yun” means no death or abortion, and “You Gu” means corresponding symptoms. However, when a severe adverse event or death occurs, the relevant TCM is not “You Gu” and should be under contraindication. This theory first indicated that appropriate administration of TCM can generate clear therapeutic effects under specific pathological status. Similarly, Zhang Jing-Yue also advocated that even toxic TCM could be used in the treatment of some diseases. Besides, modern TCM has indicated that the material basis for toxicity and that for efficacy is always the same component in cardiotoxic TCM, as mentioned above. Taking aconitine as an example, some studies show that aconitine-induced treatment is effective for rats with chronic heart failure (CHF), whereas others found that it could cause cardiac toxic effects in another kind of CHF models or healthy rats.42 These classical theories and studies imply that physical status is a possible major contributor to the transformation between TCM-generated efficacy and toxicity in organisms.
), the bible of TCM; “Wu Yun” means no death or abortion, and “You Gu” means corresponding symptoms. However, when a severe adverse event or death occurs, the relevant TCM is not “You Gu” and should be under contraindication. This theory first indicated that appropriate administration of TCM can generate clear therapeutic effects under specific pathological status. Similarly, Zhang Jing-Yue also advocated that even toxic TCM could be used in the treatment of some diseases. Besides, modern TCM has indicated that the material basis for toxicity and that for efficacy is always the same component in cardiotoxic TCM, as mentioned above. Taking aconitine as an example, some studies show that aconitine-induced treatment is effective for rats with chronic heart failure (CHF), whereas others found that it could cause cardiac toxic effects in another kind of CHF models or healthy rats.42 These classical theories and studies imply that physical status is a possible major contributor to the transformation between TCM-generated efficacy and toxicity in organisms.
Thus, it should be emphasized that unification among the organism, syndromes and efficacy/toxicity should be taken into consideration during clinical practice and preclinical research. Therefore, it is very necessary for the development and application of animal models of human diseases in the safety evaluation of cardiotoxic TCM.43 Differences in intrinsic susceptibility, biological microenvironment, and the toxic mode of action in healthy and diseased models can provide a better explanation for “physical status-efficacy/toxicity-syndrome”. Moreover, additional research will be needed to focus more on the possible mechanisms underlying the transformation between TCM-induced efficacy and toxicity and to explore the exact clinical syndrome or physical/pathological conditions suitable for the administration of these TCMs without generating any cardiotoxicity.
4.2 Safety evaluation system based on the material basis for TCM
TCM is a class of substances which are used to prevent, diagnose and heal diseases under the guidance of TCM theories. Therefore, it is not appropriate to carry out safety assessment on the cardiotoxicity of TCM independently of TCM concepts of holism and syndrome differentiation. Unfortunately, more research on TCM is performed by ignoring the traditional characters of TCM and by considering modern toxicological methods, which always indicate the potential toxic effects induced by xenobiotics. The results concluded from these studies easily promote exaggerative even incorrect knowledge of the toxicity of TCM and also cannot be helpful towards clinical treatment. In addition, the toxicity mentioned in TCM theories is often recognized as one of the natural properties of medicines and can also transform into efficacy in some cases, as documented before. Thus, developing an effective cardiotoxicity evaluation system for TCM under the guidance of TCM theories is one of the scientific problems that need to be solved urgently.Recently, studies are more prone to establish a “syndrome-toxicity-efficacy” system based on the material basis to explore the potential cardiotoxicity of TCM.44 Briefly, taking Fuzi as an example, we should first identify its indications (such as “Warming and Tonifying Spleen Yang” (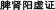 )) and its main material basis for Fuzi-induced cardiotoxicity (Aconitine). Then, animal models of this kind of heart disease or others can be applied for toxic/pharmacologic research. Later, possible molecular toxicity mechanisms and “dose-time-toxicity-efficacy” relevancy for this main material basis-induced cardiotoxicity can be well described. Besides, alterations in the dose-time-toxicity-efficacy relationship and the regularity of the efficacy and toxicity change caused by processing or combined use of Fuzi and other TCMs (such as Ginseng) can also be depicted. Lastly, all data from these studies can be conducive to the integration analysis of the possible adverse effects in different cases, standardized dosing and clinical rational drug use. All in all, after full consideration of integrated TCM theories (“Integrate disease and syndrome” (
)) and its main material basis for Fuzi-induced cardiotoxicity (Aconitine). Then, animal models of this kind of heart disease or others can be applied for toxic/pharmacologic research. Later, possible molecular toxicity mechanisms and “dose-time-toxicity-efficacy” relevancy for this main material basis-induced cardiotoxicity can be well described. Besides, alterations in the dose-time-toxicity-efficacy relationship and the regularity of the efficacy and toxicity change caused by processing or combined use of Fuzi and other TCMs (such as Ginseng) can also be depicted. Lastly, all data from these studies can be conducive to the integration analysis of the possible adverse effects in different cases, standardized dosing and clinical rational drug use. All in all, after full consideration of integrated TCM theories (“Integrate disease and syndrome” (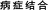 ), “prohibited combination” (
), “prohibited combination” (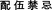 ), and “Processed detoxification” (
), and “Processed detoxification” (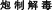 ), etc.), the cardiotoxicity of TCM under different conditions can be well modeled and presented using this safety evaluation system based on the main material basis for toxicity.
), etc.), the cardiotoxicity of TCM under different conditions can be well modeled and presented using this safety evaluation system based on the main material basis for toxicity.
4.3 Modernization strategies for toxicological research on cardiotoxic TCM
Conventional toxicology research for TCM is mainly focused on the chemical components, pharmaceutics/toxic effects and possible mechanisms, but minimal attention is given to the possible relationships among the effects, doses and time, structure–activity relationship (SAR) and the exploration of the tipping point.45,46 To detect the possible cardiotoxicity of TCM, conventional studies are preferably conducted at extremely high doses, which are much more than the real-world administration dose in a shorter term.47,48 Unluckily, most toxic TCMs always induce cardiotoxicity at very low doses without acute organic damage,47 and all the results obtained from these studies cannot monitor the early adaption/adverse responses and the material basis-induced molecular hierarchical changes in organisms, both of which are important for a complete and actual evaluation for toxic mechanisms. As a result, it is not difficult to find that conventional toxicity studies for TCM indeed exhibit good results for toxicity identification but also show remarkable deficiency in early toxicity forecast, molecular toxicology researches and the development of the relationship of “dose-time-toxicity-efficacy”.In recent years, Toxicity Testing Strategy in the 21st century (TT21C) provides a new perspective for studies on the efficacy or toxicity of TCM.49 This strategy advocates the development of related adverse outcome pathways (AOPs) based on specific toxicity pathways and focuses on the determination of quantitative SAR, dose–response assessment and the calculation of tipping points (such as the LOEAL or NOEAL value, therapeutic index) by target testing at low-level exposure or at levels of human exposure.50 Besides, incorporating a tiered approach to characterize the drugs/toxicant-induced cellular response pathway in an exposure-led framework for this next-generation risk assessment (NGRA) enables an understanding of the relevance of tipping points for adoptive/adverse responses.51,52 Therefore, toxicity studies for TCM based on this new toxicity testing strategy will not only contribute to the systematic and precise elaboration of related molecular toxicity mechanisms but also improve potential cardiotoxicity identification and scientific clinical use of these toxic TCMs.53
5. Conclusion
As a medical system with a history of more than 2000 years, TCM theories have been shown to be scientific and effective in long-term medical practice. Also, TCM has collected and summarized abundant clinical experience of TCM administration. However, there is an increase in the occurrence of TCM-induced adverse cardiac effects, which has attracted ever-growing attention all over the world and prevented the modernization and internationalization of TCM. Considering the complexity and unpredictability of TCM cardiotoxicity, in this review, we presented a simplified classification for cardiotoxic TCM and summarized the main toxic mechanisms based on the material basis for toxicity, aiming to provide the legible toxicity characters of these TCMs. Unlike chemical drugs and biological agents, the difficulties in the toxicology study of TCM mainly come from the complex components and the unpredictable drug-body interactions. After a systematic rethinking of the existing problems in previous studies on the toxicity of TCM, we emphasized the importance of a safe evaluation system for TCM based on the material basis for toxicity, which integrates new toxicity testing strategy and is launched under the guidance of TCM theories (Fig. 2).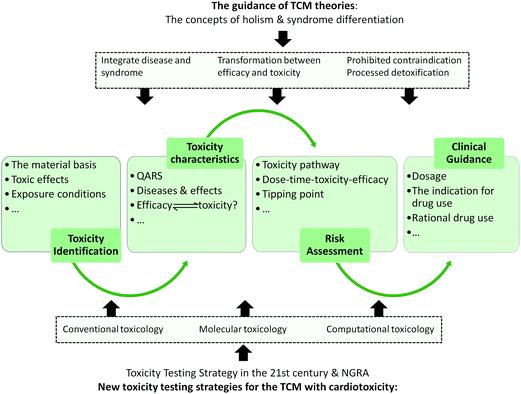 | ||
| Fig. 2 Schematic representation of the safety evaluation system integrating new toxicity testing strategies for cardiotoxic TCM under the guidance of TCM theories. | ||
Funding information
Support by the National Natural Science Foundation of China (no. 81630102 and no. 81803833).Conflicts of interest
There are no conflicts of interest to declare.References
- J. Fang, P. J. Little and S. Xu, Atheroprotective Effects and Molecular Targets of Tanshinones Derived From Herbal Medicine Danshen, Med. Res. Rev., 2018, 38, 201–228 CrossRef CAS PubMed.
- G. Zhou, L. Tang, X. Zhou, T. Wang, Z. Kou and Z. Wang, A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux, J. Ethnopharmacol., 2015, 160, 173–193 CrossRef CAS PubMed.
- R. Löbenberg, A. Dunstan, H. Castillo, G. Deol, A. Saincher, E. Yu and H. L. Banh, What Western Pharmacists Need to Know About Traditional Chinese Medicine, A Canadian Perspective, Curr. Tradit. Med., 2015, 1, 18–25 CrossRef.
- K. Chan, H. Zhang and Z. X. Lin, An overview on adverse drug reactions to traditional Chinese medicines, Br. J. Clin. Pharmacol., 2015, 80, 834–843 CrossRef PubMed.
- F. Xiong and Y. S. Guan, Cautiously using natural medicine to treat liver problems, World J. Gastroenterol., 2017, 23, 3388–3395 CrossRef PubMed.
- Y. Fan, S. Liu, X. Chen, M. Feng, F. Song and X. Gao, Toxicological effects of Nux Vomica in rats urine and serum by means of clinical chemistry, histopathology and (1)H NMR-based metabonomics approach, J. Ethnopharmacol., 2018, 210, 242–253 CrossRef PubMed.
- J. Vicente, N. Stockbridge and D. G. Strauss, Evolving regulatory paradigm for proarrhythmic risk assessment for new drugs, J. Electrocardiol., 2016, 49, 837–842 CrossRef PubMed.
- M. Zhang, C. S. Peng and X. B. Li, Human intestine and liver microsomal metabolic differences between C19-diester and monoester diterpenoid alkaloids from the roots of Aconitum carmichaelii Debx, Toxicol. In Vitro, 2017, 45, 318–333 CrossRef CAS PubMed.
- L. Zhang, J. Chang, Y. Zhao, H. Xu, T. Wang, Q. Li, L. Xing, J. Huang, Y. Wang and Q. Liang, Fabrication of a triptolide-loaded and poly-gamma-glutamic acid-based amphiphilic nanoparticle for the treatment of rheumatoid arthritis, Int. J. Nanomed., 2018, 13, 2051–2064 CrossRef CAS PubMed.
- Y. Li, J. Li, K. Zhou, J. He, J. Cao, M. An and Y. X. Chang, A Review on Phytochemistry and Pharmacology of Cortex Periplocae, Molecules, 2016, 21, 1702 CrossRef PubMed.
- X. Zhang, M. Jin, N. Tadesse, J. Dang, T. Zhou, H. Zhang, S. Wang, Z. Guo and Y. Ito, Dioscorea zingiberensis C. H. Wright: An overview on its traditional use, phytochemistry, pharmacology, clinical applications, quality control, and toxicity, J. Ethnopharmacol., 2018, 220, 283–293 CrossRef CAS PubMed.
- J. Tao, F. Jiang, C. Liu, Z. Liu, Y. Zhu, J. Xu, Y. Ge, K. Xu and P. Yin, Modulatory effects of bufalin, an active ingredient from toad venom on voltage-gated sodium channels, Mol. Biol. Rep., 2018, 45, 721–740 CrossRef CAS PubMed.
- L. A. Gonano and M. V. Petroff, Subcellular mechanisms underlying digitalis-induced arrhythmias: role of calcium/calmodulin-dependent kinase II (CaMKII) in the transition from an inotropic to an arrhythmogenic effect, Heart, Lung Circ., 2014, 23, 1118–1124 CrossRef PubMed.
- J. Li, D. Hu, X. Song, T. Han and Y. Gao, The Role of Biologically Active Ingredients from Natural Drug Treatments for Arrhythmias in Different Mechanisms, BioMed. Res. Int., 2017, 2017, 4615727 Search PubMed.
- G. Yue, Evaluation of Traditional Chinese Medicine: Foundations and Methods, Military Medical Science Press, 2017, pp. 112–124 Search PubMed.
- P. Cheng, X. Xiaohe and L. Shao, Research progress and frontier of integrated analysis of “toxicity” and “effect” of Traditional Chinese Medicine, Bull. Natl. Nat. Sci. Found. China, 2017, 31(02), 176–183 Search PubMed.
- Z. V. Varga, P. Ferdinandy, L. Liaudet and P. Pacher, Drug-induced mitochondrial dysfunction and cardiotoxicity, Am. J. Physiol. Heart Circ. Physiol., 2015, 309, H1453–H1467 CrossRef CAS PubMed.
- Y. Wang, Y. Zhao, W. Jiang, X. Zhao, G. Fan, H. Zhang, P. Shen, J. He and X. Fan, iTRAQ-Based Proteomic Analysis Reveals Recovery of Impaired Mitochondrial Function in Ischemic Myocardium by Shenmai Formula, J. Proteome Res., 2018, 17, 794–803 CrossRef CAS PubMed.
- X. Zhao, Y. Q. Shi, C. C. Yan, P. F. Feng, X. Wang, R. Zhang, X. Zhang and B. X. Li, Up-regulation of miR-21 and miR-23a Contributes to As2 O3 -induced hERG Channel Deficiency, Basic Clin. Pharmacol. Toxicol., 2015, 116, 516–523 CrossRef CAS PubMed.
- A. F. M. Botelho, A. Santos-Miranda, H. C. Joca, C. R. S. Mattoso, M. S. de Oliveira, F. Pierezan, J. S. Cruz, B. Soto-Blanco and M. M. Melo, Hydroalcoholic extract from Nerium oleander L. (Apocynaceae) elicits arrhythmogenic activity, J. Ethnopharmacol., 2017, 206, 170–177 CrossRef PubMed.
- R. J. Chen, T. R. Jinn, Y. C. Chen, T. Y. Chung, W. H. Yang and J. T. Tzen, Active ingredients in Chinese medicines promoting blood circulation as Na+/K+-ATPase inhibitors, Acta Pharmacol. Sin., 2011, 32, 141–151 CrossRef CAS PubMed.
- Y. Zhang, L. Yu, W. Jin, H. Fan, M. Li, T. Zhou, H. Wan and J. Yang, Reducing Toxicity And Increasing Efficiency: Aconitine with Liquiritin and Glycyrrhetinic Acid Regulate Calcium Regulatory Proteins in Rat Myocardial Cell, Afr. J. Tradit., Complementary Altern. Med., 2017, 14, 69–79 CrossRef CAS PubMed.
- G. B. Sun, H. Sun, X. B. Meng, J. Hu, Q. Zhang, B. Liu, M. Wang, H. B. Xu and X. B. Sun, Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats, Toxicol. Appl. Pharmacol., 2014, 279, 8–22 CrossRef CAS PubMed.
- Z. Jiawei, Mechanism of the effect of shenfu compatibility attenuation on cardiomyocytes, D,Medical University of Anhui, 2016.
- Y. Dong and J. Liao, Application of Traditional Chinese Medicine in Treatment of Atrial Fibrillation, Evid. Based Complement. Alternat. Med., 2017, 2017, 1381732 Search PubMed.
- P. Li, Q. Song, T. Liu, Z. Wu, X. Chu, X. Zhang, Y. Zhang, Y. Gao, J. Zhang and L. Chu, Inhibitory effect of cinobufagin on L-type Ca2+ currents, contractility, and Ca2+ homeostasis of isolated adult rat ventricular myocytes, Sci. World J., 2014, 2014, 496705 Search PubMed.
- J. Le He, J. W. Zhao and Z. C. Ma, et al., Cardiotoxicity study of Shenfu compatibility in rats based on metabonomics, China J. Chin. Mater. Med., 2015, 40, 2743–2747 Search PubMed.
- X. Gao, X. Zhang, J. Hu, X. Xu, Y. Zuo, Y. Wang, J. Ding, H. Xu and S. Zhu, Aconitine induces apoptosis in H9c2 cardiac cells via mitochondriamediated pathway, Mol. Med. Rep., 2018, 17, 284–292 CAS.
- J. Zhou, C. Xi, W. Wang, X. Fu, L. Jinqiang, Y. Qiu, J. Jin, J. Xu and Z. Huang, Triptolide-induced oxidative stress involved with Nrf2 contribute to cardiomyocyte apoptosis through mitochondrial dependent pathways, Toxicol. Lett., 2014, 230, 454–466 CrossRef CAS PubMed.
- E. Haque, S. Irfan, M. Kamil, S. Sheikh, A. Hasan, A. Ahmad, V. Lakshmi, A. Nazir and S. Mir, Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae, 2016 Search PubMed.
- C. Pace, R. Dagda and J. Angermann, Antioxidants Protect against Arsenic Induced Mitochondrial Cardio-Toxicity, Toxics, 2017, 5, 38 CrossRef PubMed.
- K. S. Lee, A. Kronbichler, M. Eisenhut, K. H. Lee and J. I. Shin, Cardiovascular involvement in systemic rheumatic diseases: An integrated view for the treating physicians, Autoimmun. Rev., 2018, 17, 201–214 CrossRef PubMed.
- M. Yi, W. Peng, X. Chen, J. Wang and Y. Chen, Effect of hypaconitine combined with liquiritin on the expression of calmodulin and connexin43 in rat cardiac muscle in vivo, J. Pharm. Pharmacol., 2012, 64, 1654–1658 CrossRef CAS PubMed.
- D. H. Hu, Y. G. Wang and C. Z. Wu, Effect of Panax notoginseng saponin on cytochrome P450 mRNA expression in H9c2 cell, Chin. Pharmacol. Bull., 2013, 29, 1563–1567 Search PubMed.
- R. S. Jia, X. H. Hua and L. Ming, et al., Cytotoxicity of ophiopogonin D′ for rat H9c2 cardiomyocytes, Chin. J. Pharmacol. Toxicol., 2017, 31, 325–331 Search PubMed.
- Y. Wang, T. Han, L. M. Xue, P. Han, Q. Y. Zhang, B. K. Huang, H. Zhang, Q. L. Ming, W. Peng and L. P. Qin, Hepatotoxicity of kaurene glycosides from Xanthium strumarium L. fruits in mice, Pharmazie, 2011, 66, 445–449 CAS.
- F. Bucaretchi, E. M. De Capitani, M. M. Branco, L. C. Fernandes and S. Hyslop, Coagulopathy as the main systemic manifestation after envenoming by a juvenile South American rattlesnake (Crotalus durissus terrificus): case report, Clin. Toxicol., 2013, 51, 505–508 CrossRef CAS PubMed.
- G. Frommeyer, J. Weller, C. Ellermann, N. Bogeholz, P. Leitz, D. G. Dechering, S. Kochhauser, K. Wasmer and L. Eckardt, Ivabradine Reduces Digitalis-induced Ventricular Arrhythmias, Basic Clin. Pharmacol. Toxicol., 2017, 121, 526–530 CrossRef CAS PubMed.
- J. J. Wu, Z. Z. Guo, Y. F. Zhu, Z. J. Huang, X. Gong, Y. H. Li, W. J. Son, X. Y. Li, Y. M. Lou, L. J. Zhu, L. L. Lu, Z. Q. Liu and L. Liu, A systematic review of pharmacokinetic studies on herbal drug Fuzi: Implications for Fuzi as personalized medicine, Phytomedicine, 2018, 44, 187–203 CrossRef PubMed.
- G. Patti, I. Cavallari, O. Hanon and R. De Caterina, The safety and efficacy of non-vitamin K antagonist oral anticoagulants in atrial fibrillation in the elderly, Int. J. Cardiol., 2018, 265, 118–124 CrossRef PubMed.
- I. D. de Souza, A. S. de Andrade and R. J. S. Dalmolin, Lead-interacting proteins and their implication in lead poisoning, Crit. Rev. Toxicol., 2018, 48, 375–386 CrossRef PubMed.
- B. Yu, Y. Cao and Y. K. Xiong, Pharmacokinetics of aconitine-type alkaloids after oral administration of Fuzi (Aconiti Lateralis Radix Praeparata) in rats with chronic heart failure by microdialysis and ultra-high performance liquid chromatography-tandem mass spectrometry, J. Ethnopharmacol., 2015, 165, 173–179 CrossRef CAS PubMed.
- N. Ke-Yong and L. Min, Application of morbid animal model in drug safety evaluation of traditional Chinese medicine, Front. Pharmacol., 2015, 6, 37 Search PubMed.
- J. Shen, J. Wang, E. X. Shang, Y. P. Tang, J. Kai, Y. J. Cao, G. S. Zhou, W. W. Tao, A. Kang, S. L. Su, L. Zhang, D. W. Qian and J. A. Duan, The dosage-toxicity-efficacy relationship of kansui and licorice in malignant pleural effusion rats based on factor analysis, J. Ethnopharmacol., 2016, 186, 251–256 CrossRef CAS PubMed.
- M. Zhang, J. Wang, L. Zhu, T. Li, W. Jiang, J. Zhou, W. Peng and C. Wu, Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology, 2017, 18 Search PubMed.
- Y. Guo, T. Yin, X. Wang, F. Zhang, G. Pan, H. Lv, X. Wang, J. Owoicho Orgah, Y. Zhu and H. Wu, Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review, J. Ethnopharmacol., 2017, 209, 264–282 CrossRef CAS PubMed.
- J. H. Zhang, H. L. Xin, Y. M. Xu, Y. Shen, Y. Q. He, Y. Hsien, B. Lin, H. T. Song, L. Juan, H. Y. Yang, L. P. Qin, Q. Y. Zhang and J. Du, Morinda officinalis How. - A comprehensive review of traditional uses, phytochemistry and pharmacology, J. Ethnopharmacol., 2018, 213, 230–255 CrossRef CAS PubMed.
- P. Kubincová, J. Novák and I. Sovadinová, Acute Systemic Toxicity: Alternative in Vivo and in Vitro Methods, 2016 Search PubMed.
- A. R. Iskandar, C. Mathis, F. Martin, P. Leroy, A. Sewer, S. Majeed, D. Kuehn, K. Trivedi, D. Grandolfo, M. Cabanski, E. Guedj, C. Merg, S. Frentzel, N. V. Ivanov, M. C. Peitsch and J. Hoeng, 3-D nasal cultures: Systems toxicological assessment of a candidate modified-risk tobacco product, Altex, 2017, 34, 23–48 CrossRef PubMed.
- K. L. Dearfield, B. B. Gollapudi, J. C. Bemis, R. D. Benz, G. R. Douglas, R. K. Elespuru and G. E. Johnson, Next generation testing strategy for assessment of genomic damage: A conceptual framework and considerations, Environ. Mol. Mutagen., 2017, 58, 264–283 CrossRef CAS PubMed.
- I. Cote, P. T. Anastas, L. S. Birnbaum, R. M. Clark, D. J. Dix, S. W. Edwards and P. W. Preuss, Advancing the next generation of health risk assessment, Environ. Health Perspect., 2012, 120, 1499–1502 CrossRef PubMed.
- A. Middleton, S. Cooper, T. Cull, R. Stark, Y. Adeleye, K. Boekelheide, R. Clewell, P. Jennings, J. Guo and C. Liu, Case Studies in Cellular Stress: Defining Adversity/Adaptation Tipping Points, Appl. In Vitro Toxicol., 2017, 3, 199–210 CrossRef.
- D. Q. Chen, H. Chen, L. Chen, D. D. Tang, H. Miao and Y. Y. Zhao, Metabolomic application in toxicity evaluation and toxicological biomarker identification of natural product, Chem.-Biol. Interact., 2016, 252, 114–130 CrossRef CAS PubMed.
Footnote |
| † Equal contributor. |
| This journal is © The Royal Society of Chemistry 2019 |