Realizing an impressive red-emitting Ca9MnNa(PO4)7 phosphor through a dual function based on disturbing structural confinement and energy transfer†
Jindi
Wang
a,
Mengmeng
Shang
 *a,
Min
Cui
a,
Peipei
Dang
b,
Dongjie
Liu
b,
Dayu
Huang
b,
Hongzhou
Lian
b and
Jun
Lin
*a,
Min
Cui
a,
Peipei
Dang
b,
Dongjie
Liu
b,
Dayu
Huang
b,
Hongzhou
Lian
b and
Jun
Lin
 *b
*b
aSchool of Chemistry and Chemical Engineering, Qingdao University, Qingdao 266071, P. R. China. E-mail: mmshang@qdu.edu.cn
bState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: jlin@ciac.ac.cn
First published on 20th November 2019
Abstract
In the field of white LEDs, the research and development of red phosphors have been confronting a challenge. In this paper, a novel red-emitting Ca9MnNa(PO4)7 (CMNPO) phosphor, whose emission was attributed to the 4T1(4G)–6A1(6S) transition of Mn2+ ions, was prepared by a high-temperature solid-state reaction. We utilized a chemical co-substitution strategy to optimize the luminescence properties and realize an impressive red emission in our CMNPO phosphor. The introduction of Zn2+/Mg2+ into CMNPO could break the intrinsic structural confinement of Mn2+, which improved the red emission from Mn2+ in the narrow confines. The novelty of this work lies in realizing an efficient red phosphor by doping trace amounts of Eu2+. By partially replacing Mn2+ ions with trace amounts of Eu2+, we obtained a CMNPO phosphor that emitted a dazzling red light, and the quantum yield reached up to 82%. The intensity dependence of this red emission on the trace amounts of doped Eu2+ was quantitatively analyzed. The substitution of Eu2+ ions played a dual role in improving the luminescence properties, not only disturbing the structural confinement of Mn2+ but also transferring energy to Mn2+ efficiently. By employing it as a red phosphor, we fabricated various high-performance white LEDs with low correlated color temperatures (4642–4956 K) and high-color-rendering indices (80.5–83.4). These findings show the potential promise of the CMNPO phosphor as a red phosphor in warm white LEDs and open up new avenues for the exploration of novel red-emitting phosphors.
Introduction
According to statistics, lighting accounts for approximately 20 percent of the global electricity consumption, one-third of which is expended by poorly efficient incandescent lamps.1–3 Accordingly, this waste of electricity has aroused increasing interest in using white light-emitting devices (LEDs) due to their low cost, high throughput, and significant efficiency compared to incandescent light sources and fluorescent lamps.4,5 In recent years, the superiority of white light-emitting diodes (LEDs) has promoted their applications in lighting and displays, incorporating them into people's daily lives.6–11 Nowadays, commercially available white LED devices are manufactured by depositing the yellow-emitting Y3Al5O12:Ce3+ (YAG:Ce3+) phosphor on a blue LED chip. Albeit the structural simplicity, high luminous efficiency, and low cost,12–14 there are some challenges in this kind of white LEDs that need to be solved urgently. For example, these devices are not suitable for residential applications as the white emitted light lacking the red component has an undesirable color balance, which makes it challenging to efficiently reproduce real colors.15 On the one hand, one solution is to supplement the missing red component by adding an appropriate red phosphor to the commercially available white LED device. On the other hand, using an ultraviolet LED chip pumping red, green and blue (RGB) three-color phosphors to output white light is another promising approach.16,17 It can be seen that in all these methods, there is an urgent need for a suitable red phosphor.18 Based on previous reports, the ideal red phosphor should have some specific characteristics such as a broad excitation band in the near-UV and blue light region, a narrow red emission band, high luminous efficiency, and high conversion efficiency under the LED's operating conditions.19,20 At present, based on versatile structural models, a large number of red-emitting phosphor materials have been developed. Among them, Eu2+-doped nitrides, such as (Ba,Sr)2Si5N8:Eu2+ and (Ca,Sr)SiAlN3:Eu2+,21–25 as well as Mn4+-doped fluorides, such as K2SiF6:Mn4+,26 have drawn much attention. However, nitride phosphors require harsh preparation conditions and display a higher re-absorption effect among phosphors, and fluoride phosphors are susceptible to the moisture in air, all of which inspired us to explore new red-light materials with excellent properties.27–29 As a kind of important luminescent materials, phosphate compounds have attracted much attention due to their excellent stability and mild synthesis conditions. In this work, we chose a β-Ca3(PO4)2-type mineral structure as a structural prototype since it can be tuned to obtain multifarious derivatives owing to the possibility of the heterovalent substitution of cations.29 In recent years, a large number of novel β-Ca3(PO4)2-type phosphors have been designed and synthesized, such as Eu2+ and Mn2+ co-doped Ca8ZnCe(PO4)7 phosphors, Ca8ZnLu(PO4)7 phosphors, and (Ca,Sr)9Sc(PO4)7 phosphors.30–40 It is worth noting that previous studies are mainly based on the energy transfer between two doping ions to achieve multiple colors in single-phase phosphors. However, the design of an efficient red phosphor using energy transfer has rarely been reported.41 Drawing inspiration from the above reasons, we developed a new β-Ca3(PO4)2-type compound with the nominal chemical formula of Ca9MnNa(PO4)7, which could emit pure red light. The novelty of our work lies in realizing an efficient red phosphor by doping trace amounts of Eu2+. In this work, the structure and properties of this phosphor were studied. Furthermore, we utilized a widely acceptable chemical substitution strategy to optimize the luminescence properties and realized an impressive red emission from this Ca9MnNa(PO4)7 phosphor.42,43 The rare earth Eu2+ ions partially substituted Mn2+ ions in the original host crystal lattice and played a pivotal role in enhancing the red luminescence intensity of Mn2+ because they acted as blockers interrupting Mn–Mn energy transfer and as sensitizers transferring energy to Mn2+. At last, we realized warm white LEDs with low CCT and high CRI by employing Eu2+-substituted Ca9MnNa(PO4)7 as the red phosphor. All the experimental results present a substantial advancement towards the commercial application of LEDs.Experimental
Materials and synthesis
Phosphors with the nominal compositions of Ca9Mn1−zNa(PO4)7:zEu2+ (abbreviated as CMNPO:zEu2+, 0 ≤ z ≤ 0.9) were synthesized by a high-temperature solid-state method. The raw materials were CaCO3 (A.R.), Na2CO3 (A.R.), MnCO3 (A.R.), NH4H2PO4 (A.R.) and Eu2O3 (99.99%). The typical preparation process is as follows. First, the raw materials were weighed according to the target stoichiometric ratio and mixed in an agate mortar with absolute ethyl alcohol. Next, the mixtures were fully ground, placed in a crucible and then preheated at 750 °C for 3 h. After being ground repeatedly, the obtained powders were sintered again at 1300 °C for 5 h in a tube furnace under a reducing atmosphere of 90% N2/10% H2. After thorough grinding, the powders for further analysis were finally obtained and showed red luminescence when excited with a 365 nm UV lamp. The powders were then used for further analysis.The preparation processes for the Ca9Mn1−xNa(PO4)7:xZn2+ and Ca9Mn1−yNa(PO4)7:yMg2+ samples were the same as that for CMNPO:zEu2+ except that the corresponding raw materials, i.e., ZnO and MgO were used instead of Eu2O3.
Characterization
Phase purity and crystal structure identification of the as-prepared products were studied on a Bruker D8 diffractometer operated at 40 kV and 40 mA at a scanning rate of 1° min−1 with a Cu Kα source (2θ = 10–120°, λ = 0.1541 nm). The obtained XRD data were refined by the Rietveld method in General Structure Analysis System (GSAS) software,44 and the crystal structure diagram was drawn with the CrystalMaker Demo software. The diffuse reflection spectra were recorded by a Hitachi U-4100 UV-vis-NIR spectrophotometer and photoluminescence quantum yields (QY) were measured by the absolute PL quantum yield (internal quantum efficiency) measurement system (C9920-02, Hamamatsu Photonics). Room temperature photoluminescence (PL) spectra and photoluminescence excitation spectra (PLE) were measured on an Edinburgh FS5 fluorescence spectrometer equipped with a 150 W xenon lamp as the excitation source. The temperature-dependent (−190 to 300 °C) PL spectra were obtained on a fluorescence spectrophotometer (Edinburgh Instruments FLSP-920) equipped with a temperature controller. The luminescent photographs of the samples were captured by a CCD camera, and the morphology and elemental composition of the samples were studied by scanning electron microscopy (FE-SEM, S-4800, Hitachi). The luminescence lifetimes were recorded with a LeCroy Wave Runner 6100 digital oscilloscope (1 GHz) using a tunable laser (pulse width 4 ns, gate 50 ns) as the excitation source (Continuum Sunlite OPO).Results and discussion
Identification of phases and components
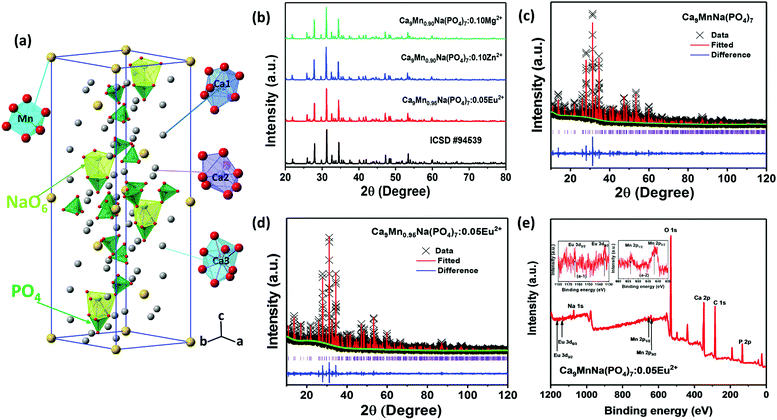
β-Ca3(PO4)2-type phosphors have been extensively investigated in recent years. As reported, β-Ca3(PO4)2 has a rhombohedral structure with the R3c space group and has five Ca(I) (I = 1, 2, 3, 4, and 5) cationic sites. The average bond lengths of Ca(I)–O are 2.43, 2.48, 2.543, 2.531, and 2.265 Å.45,46 Moreover, β-Ca3(PO4)2 can also be expressed as β-Ca21(PO4)14. When two divalent A2+ ions (A = Mn, Ba, Mg) and two monovalent B+ ions (B = H, Li, Na) are substituted for three divalent Ca2+ ions, β-Ca18A2B2(PO4)14 can be obtained, which is equivalent to Ca9AB(PO4)7. Therefore, in this work, Ca9MnNa(PO4)7 is designated as a β-Ca3(PO4)2-type structure. Fig. 1a shows the structural view of CMNPO and the coordination environments for Ca and Mn are highlighted. In the CMNPO host, there are three independent cationic sites, namely, Ca(1), Ca(2) and Ca(3). The coordination number of the three Ca atoms is 8 or 9, and the average bond length is 2.505 Å. Mn2+ ions occupy the six-coordinated crystal lattice position, forming an octahedron with six oxygen atoms, which can determine the luminescence properties of CMNPO. Fig. 1b shows the XRD patterns for the representative CMNPO:0.05Eu2+, CMNPO:0.10Zn2+ and CMNPO:0.10Mg2+ phosphors along with the reference diffraction lines based on the simulated patterns found in ICSD #94539. By the comparison of the XRD profiles, the positions and intensities of the diffraction peaks for all samples are consistent with the standard pattern of Ca9MnNa(PO4)7, and no impurity phases are observed. This indicates that all doped ions have been well dissolved in the host, maintaining the phase purity. To further study the phase purity and crystal structure of the CMNPO and CMNPO:0.05Eu2+ phosphors, the Rietveld refinement was performed on the powder X-ray data, as shown in Fig. 1c and d. The red solid lines and the black crosses show the calculated data and the experimental data, respectively. The difference between the experimental data and the calculated data is indicated by the blue line at the bottom. The refined residual factors, cell parameters, and fractional atomic coordinates are shown in Tables S1–S4 (ESI†). The unit cell parameters of CMNPO are a = b = 10.39225 Å, c = 37.1362 Å, and V = 3473.33 Å3, and the unit cell parameters of CMNPO:0.05Eu2+ are a = b = 10.39560 Å, c = 37.1523 Å, and V = 3477.09 Å3. When Eu2+ is doped, the cell parameters become larger, which is attributed to the larger radius of Eu2+ (r = 1.25 Å, CN = 8) than that of Mn2+ (r = 0.96 Å, CN = 8). Based on the refined data, the cell parameters of our CMNPO and CMNPO:0.05Eu2+ are found to be close to those of standard CMNPO. These results further indicate that the synthesized samples contain pure-phase crystals, which have a β-Ca3(PO4)2-type structure. The elemental compositions and electronic states of the samples were determined by XPS. The XPS analysis confirmed the presence of all the elements expected in the CMNPO:Eu2+ sample, as shown in Fig. 1e. Moreover, as demonstrated in the insets (a-1 and a-2 in Fig. 1e), the binding energies of around 1166.8, 1135.6 eV and 653, 641 eV indicate the presence of divalent Eu2+ 3d3/2, 3d5/2 and Mn2+ 2p1/2, 2p3/2 core peaks, respectively.47,48In order to further study the morphology and composition of the as-synthesized samples, SEM and elemental mapping were performed. The SEM image of the representative CMNPO:0.05Eu2+ sample is shown in Fig. 2a. Obviously, the surface of the sample is relatively irregular, and the particle size is 1–5 μm. Fig. 2b presents the elemental maps of the selected typical microcrystalline particles. The analysis results indicate that the Ca, Mn, Na, P, O and Eu elements present in the sample are uniformly distributed.
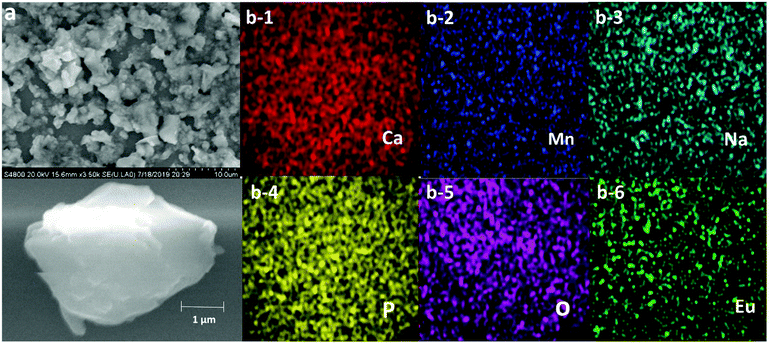 | ||
| Fig. 2 (a) SEM image of Ca9Mn0.95Na(PO4)7:0.05Eu2+ microcrystal particles and (b) the elemental mapping images of Ca, Mn, Na, P, O and Eu. | ||
Impressive enhancement of red emission in Ca9MnNa(PO4)7 phosphor
Next, to investigate the luminescence properties, the diffuse reflectance (DR) spectra of the CMNPO and CMNPO:0.10Eu2+ phosphors were first characterized, as shown in Fig. 3. The optical band gap of CMNPO could be calculated by the following absorption function:49,50| F(R) = (1 − R)2/2R |
| [F(R)hν]1/2 = A(hν − Eg) | (1) |
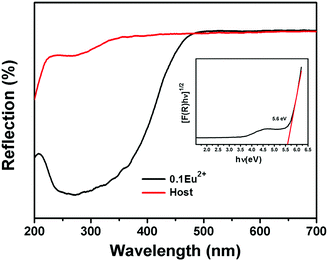 | ||
| Fig. 3 Diffuse reflectance spectra of the Ca9MnNa(PO4)7 host (red line) and Ca9Mn0.90Na(PO4)7:0.10Eu2+ sample (black line). The inset presents the band gap energy for the Ca9MnNa(PO4)7 host. | ||
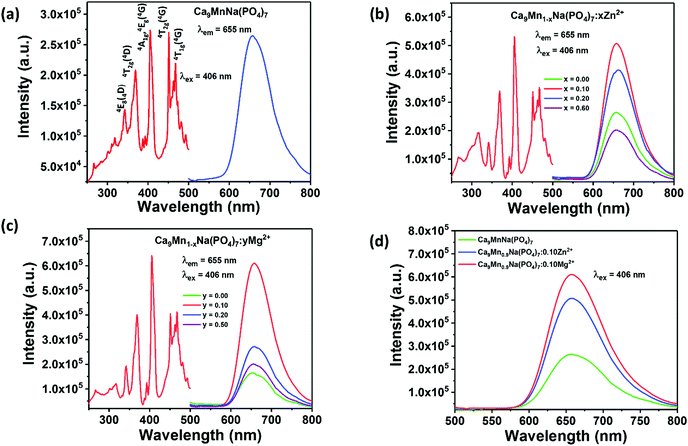
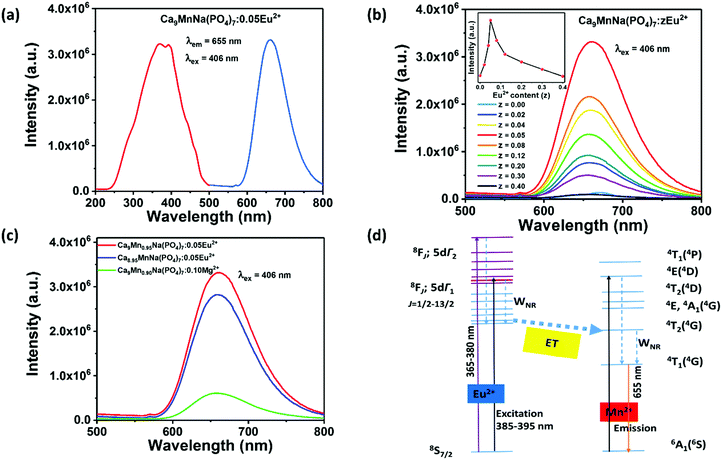
Since the observed 3d–3d transitions are both parity- and spin-forbidden, the intrinsic excitation of the Mn2+ ions is very weak and has poor efficiency. Therefore, it is limited to enhance the red emission of the CMNPO phosphor only through breaking the structural confinement.54,57 Eu2+ is a well-known sensitizer for Mn2+ ions, and many color-tunable phosphors, for example, Eu2+ and Mn2+ co-doped Sr3LuNa(PO4)3F and K2BaCa(PO4)2 phosphors based on ET between Eu2+ and Mn2+ have been reported.58,59 Hence, Eu2+ ions were introduced as sensitizers to improve the PL emission. Fig. 5a shows the excitation spectrum of the CMNPO:0.05Eu2+ phosphor monitored at 655 nm and the emission spectrum at 406 nm excitation. Different from the result for pure CMNPO, the PLE spectrum of CMNPO:0.05Eu2+ presents a strong excitation band with a maximum at 390 nm in the range from 260 to 450 nm, which can be assigned to the transitions of the Eu2+ ions from the 4f7 ground state to the crystal-field split 4f6–5d1 configuration, indicating efficient energy transfer between Eu2+ and Mn2+.60 What needs to be specifically stated is that the optimal excitation wavelength of CMNPO is 406 nm. Therefore, in order to better compare the emission intensities under the same conditions, 406 nm (not 390 nm) was used as the excitation wavelength in our work. The photoluminescence spectrum of CMNPO:0.05Eu2+ shows only one red emission peak at about 655 nm, originating from the 4T1(4G) → 6A1(6S) transition of Mn2+. Fig. 5b presents the concentration-dependent PL spectra of the CMNPO:zEu2+ (0 ≤ z ≤ 0.4) phosphors excited at 406 nm, and the PL intensity dependence on the concentration of the Eu2+ ions is also shown. Moreover, the PL spectra for all the prepared CMNPO:zEu2+ (0 ≤ z ≤ 0.9) samples are given in Fig. S3 (ESI†). It can be clearly seen that the emission intensity of CMNPO:zEu2+ increases first and then decreases with the increase in the Eu2+ doping concentration, but the peak shape of the emission spectrum does not change, which indicates that Eu2+ is stable in the crystal structure of CMNPO. Furthermore, the PL intensity increases sharply with the introduction of the Eu2+ ions and reaches a maximum for z = 0.05, which is 19 times stronger than that of the pure host. The quantum yield for the CMNPO:0.05Eu2+ sample is as high as 82%, which is quite valuable for exploring the use of the Mn2+-based red phosphors for phosphor-converted white LED applications. This enhancement in the PL intensity can be explained taking into account the high-efficiency ET process between the Eu2+ and Mn2+ ions. The quenching behavior (when z exceeds 0.05) is attributed to the fact that on increasing the Eu2+ doping concentration, the distance between two Eu2+ sensitizer ions is gradually reduced, and the ET between Eu-Eu is enhanced. On the one hand, the increased energy transfer rate between Eu–Eu competes with the ET from Eu2+ to Mn2+ and finally reduces the ET for Eu–Mn. On the other hand, when the doping concentration increases to a certain extent, concentration quenching occurs, and the luminescence of Eu2+ is weakened, which reduces the energy transfer from Eu2+ to Mn2+.51,61 In the CMNPO:0.05Eu2+ phosphor, although the energy transfer between Mn–Mn reduces the population of the luminescent 4T1(4G) state, the structural constraint makes the Eu–Mn ET process more efficient, which provides a pretty strong pumping behavior and significantly enhances the red emission of Mn2+.51,61 Due to this highly efficient energy transfer process, the characteristic emission of Eu2+ in CMNPO:zEu2+ is too weak to be observed.41Fig. 5c further compares the effects of the broken structural confinement and energy transfer on PL intensity and exhibits the emission spectra of Ca9Mn0.95Na(PO4)7:0.05Eu2+, Ca8.95MnNa(PO4)7:0.05Eu2+, and Ca9Mn0.90Na(PO4)7:0.10Mg2+. In Ca9Mn0.95Na(PO4)7:0.05Eu2+, Eu2+ ions partially substitute Mn2+, breaking the structural confinement due to the high concentration of Mn2+ and simultaneously transferring their energy to Mn2+. However, there is only one function in the Ca8.95MnNa(PO4)7:0.05Eu2+ and Ca9Mn0.90Na(PO4)7:0.10Mg2+ samples. In the former, Eu2+ ions partially replace Ca2+ and only energy transfer plays a vital role. In the latter, Mg2+ is an optically inert ion and only plays a role in breaking the structural confinement. Thus, Fig. 5c demonstrates that the Ca9Mn0.95Na(PO4)7:0.05Eu2+ phosphor shows the strongest PL intensity. Furthermore, for the sake of verifying the effect of Mg2+/Zn2+ on the luminescence of the Ca9Mn0.95Na(PO4)7:0.05Eu2+ phosphor, the PL spectra of the Ca9Mn0.95Na(PO4)7:0.05Eu2+, Ca9Mn0.85Mg0.10Na(PO4)7:0.05Eu2+ and Ca9Mn0.85Zn0.10Na(PO4)7:0.05Eu2+ phosphors were compared, as shown in Fig. S4 (ESI†). Obviously, the Ca9Mn0.95Na(PO4)7:0.05Eu2+ sample exhibits the strongest emission and doping with Mg2+/Zn2+ reduces the luminescence of Ca9Mn0.95Na(PO4)7:0.05Eu2+. This is because compared with the observation for the Ca9Mn0.85B0.10Na(PO4)7:0.05Eu2+ (B = Mg or Zn) phosphors, the concentration of Mn2+ in the Ca9Mn0.95Na(PO4)7:0.05Eu2+ sample is optimal and can help achieve the best emission intensity. More importantly, this result from another perspective proves that a high Mn concentration is essential to red emission, and the highly efficient ET between Eu–Mn is the key factor for successfully obtaining an impactful red phosphor. Therefore, a simple energy level diagram is given in Fig. 5d, depicting the energy transfer between Eu2+ and Mn2+. As shown, in the CMNPO:Eu2+ sample, the Eu2+ ions in the crystal lattice absorb ultraviolet radiation, and a majority of the excitation energy is transferred from Eu2+ to the excited state of Mn2+, which significantly enhances the red light emission of Mn2+.
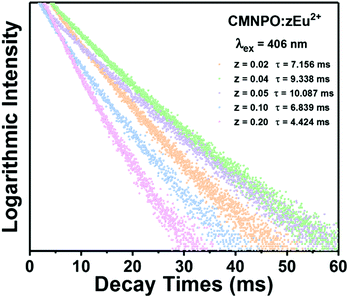
The decay curves and lifetimes of the Mn2+ emission at 655 nm in a series of CMNPO:zEu2+ phosphors (z = 0.02–0.2) were measured, as shown in Fig. 6. The decay curves were well fitted with the following second-order exponential equation:62,63
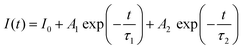 | (2) |
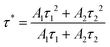 | (3) |
On the basis of the above equation, the average lifetimes of the Mn2+ emission in CMNPO:zEu2+ (z = 0.02, 0.04, 0.05, 0.10, and 0.20) were determined to be 7.156, 9.338, 10.087, 6.839, and 4.424 ms, respectively. These changes in the lifetimes with concentration are consistent with the PL dependence, as shown in Fig. 5b. The observed dependence of the Mn2+ lifetimes on Eu2+ concentration indicates that a double effect on the occupation number of the 4T1(4G) state of Mn2+ ions originated from the ET process from Eu to Mn is greater than that of the spontaneously radiative process of Mn2+ ions. As a result, the higher the energy efficiency between Eu and Mn, the longer the average lifetime of Mn2+.
Thermal quenching properties and LED applications of CMNPO:zEu2+ phosphors
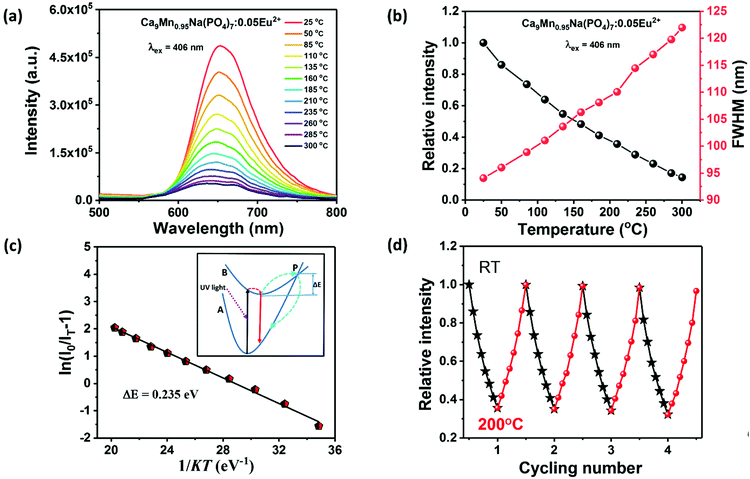
Since the operating temperature of WLEDs is usually around 150 °C, the thermal quenching property of phosphors is one of the extremely significant parameters for ensuring the high efficiency and color stability of phosphor-converted high-power white LEDs.64–66 Under the excitation of 406 nm, the temperature-dependent PL spectra of CMNPO:0.05Eu2+ from 25 °C to 300 °C were characterized and are given in Fig. 7a. The variations in the emission intensity and full width at half maximum (FWHM) of the CMNPO:0.05Eu2+ phosphor as functions of temperature are given in Fig. 7b. As shown, the luminescence intensity decreases on increasing the temperature. The luminescence intensity at 150 °C is 50% of the initial intensity, which indicates that Mn2+ is sensitive to temperature changes in the CMNPO matrix. We can also find that the FWHM of CMNPO:0.05Eu2+ increases with the increase in temperature. The increase in FWHM and the decrease in emission intensity can be explained by thermal quenching, as shown in the configuration coordinate diagram.23,67,68 It can be simply described that the excited electrons can overcome the energy barrier and spread to high energy vibration levels with the increase in temperature. The radiative transitions between these energy levels lead to the broadening of the spectral emission band.To further discuss the relationship between photoluminescence and temperature, we calculated the activation energy of temperature-related thermal quenching and fitted the thermal quenching data using the Arrhenius equation:69–71
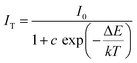 | (4) |
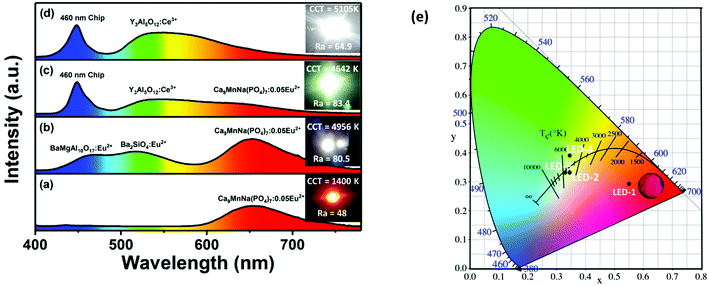
Finally, in order to evaluate the potential application of CMNPO:Eu2+ as a red phosphor component in white LED devices, we first fabricated an LED device (labeled as LED-1) by combining a UV-LED chip (370 nm) and the red CMNPO:0.05Eu2+ phosphor. The corresponding electroluminescence (EL) spectrum is given in Fig. 8a. Moreover, the position of the CMNPO:0.05Eu2+ phosphor excited at 370 nm in the Commission International de I’éclairage (CIE) chromaticity diagram was calculated, and a digital luminescence photograph under a 365 nm UV lamp is also shown (Fig. 8e). The above experimental observations indicate that the CMNPO:0.05Eu2+ phosphor shows a bright red emission with the CIE coordinates of (0.5517, 0.2921), and the fabricated red LED device exhibits an excellent performance. Then, on the one hand, we used red-emitting CMNPO:0.05Eu2+, green-emitting Ba2SiO4:Eu2+ and blue-emitting BaMgAl10O17:Eu2+ as trichromatic phosphors and combined them with UV-LED chips (370 nm) to fabricate a white LED device (LED-2). The corresponding electroluminescence spectra driven by a 100 mA current, photos, CCT and Ra are shown in Fig. 8b. The CIE color coordinates of the fabricated white LED are located at (0.3472, 0.3309) (Fig. 8e) and the correlated color temperature (CCT) value is 4956 K. The color rendering index value (Ra) reaches 80.5. All these results indicate that a bright warm white light was obtained from the fabricated device, and CMNPO:0.05Eu2+ has a great potential to serve as the red phosphor of UV-pumped white LEDs. On the other hand, as indicated in Fig. 5a, the as-prepared CMNPO:0.05Eu2+ phosphor can be readily excited by a blue light at 460 nm. As we all know, dominantly white LEDs are produced by a blue-emitting chip covered with a commercial YAG:Ce3+ yellow-emitting phosphor. Unfortunately, such white LEDs produce a cool white light with high CCT and low CRI due to the insufficient red light emission of their components. Thus, we also fabricated an LED that produced white light (Ra = 83.4, CCT = 4642 K) by combining 460 nm blue chips, commercial YAG:Ce3+ yellow phosphor and the as-prepared CMNPO:0.05Eu2+ red phosphor (LED-3). We also prepared a typical white LED device with blue chips and commercial YAG:Ce3+ yellow phosphor as a reference (LED-4).
The corresponding electroluminescence spectra driven by a 100 mA current are shown in Fig. 8c and d. It can be seen through comparing LED-3 and LED-4 that a pronounced warmer tone of the emitting light was observed in the emission spectrum of LED-3 owing to the increased red light component. CCT decreased from 5105 K to 4642 K, and the Ra value was greatly improved. The CIE color coordinates for LED-3 and LED-4 are located at (0.3299, 0.3317) and (0.3451, 0.3902), respectively. The color points of LED-3 are much closer to the black body locus than those of LED-4. In addition, the CIE coordinates, CCT, Ra and luminous efficiency (LE) of LED1–4 are given in Table S5 (ESI†). Compared with the previously reported single-phase β-Ca3(PO4)2-type Ca8ZnLu(PO4)7:Eu2+,Mn2+ phosphor (Ra = 88.6, CCT = 4842 K), the as-prepared white LED devices showed lower CCT, which may be more suitable for indoor lighting.40 However, the CMNPO red phosphor still has some flaws compared with some fluoride/nitride red phosphors.21–23,26
Conclusions
In this work, a new red-emitting CMNPO phosphor with a whitlockite-type structure was synthesized by a high-temperature solid-state reaction. The weak and inefficient excitation from the spin- and parity-forbidden Mn2+ d–d transition led to a poor-efficiency red emission. We utilized a chemical co-substitution strategy to optimize the luminescence properties and realized an impressive red emission in the CMNPO phosphor. The introduction of Zn2+/Mg2+ into CMNPO could break the intrinsic structural confinement of Mn2+, which improved the red emission of Mn2+ in the narrow confines. However, the CMNPO phosphor when doped with Eu2+ emitted a dazzling red light. The quantum yield for the CMNPO:0.05Eu2+ sample was as high as 82%, which was 19 times higher than that of the pure CMNPO host. Different from the Zn2+/Mg2+ ions, the Eu2+ ions played a role as sensitizers, and the intrinsic highly efficient Eu–Mn ET process was critical to achieve an effective red emission. More importantly, this phosphor exhibited excellent thermal cycling stability. Due to the existence of Eu2+ ions, the excitation band of CMNPO:0.05Eu2+ in the range of 250–500 nm perfectly matched with UV and blue LED chips. Finally, by integrating the CMNPO:0.05Eu2+ phosphor with commercial green-emitting Ba2SiO4:Eu2+, blue-emitting BaMgAl10O17:Eu2+ and a 370 nm UV-LED chip, we fabricated a warm UV-white LED with high CRI (Ra = 80.5) and relatively low CCT (4956 K). Moreover, by integrating this red phosphor with the commercial YAG:Ce3+ yellow phosphor and a 460 nm blue chip, an improved white LED device with lower CCT and higher Ra was obtained.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC 51672265), the China Postdoctoral Science Foundation (2018M630748), the Natural Science Foundation of Shandong Province (ZR2018JL016), the Applied Basic Research Plan of Qingdao (18-2-2-15-jch) and the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization (RERU2019003).References
- Y. R. Sun, N. C. Giebink, H. Kanno, B. W. Ma, M. E. Thompson and S. R. Forrest, Nature, 2006, 440, 908 CrossRef CAS.
- B. W. D'Andrade and S. R. Forrest, Adv. Mater., 2004, 16, 1585 CrossRef.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, M. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. H. Sargent and J. Tang, Nature, 2018, 563, 7732 CrossRef.
- H. Cho, S.-H. Jeong, M.-H. Park, Y.-H. Kim, C. Wolf, C.-L. Lee, J. H. Heo, A. Sadhanala, N. Myoung, S. Yoo, S. H. Im, R. H. Friend and T.-W. Lee, Science, 2015, 350, 1222 CrossRef CAS.
- Z.-K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687 CrossRef CAS.
- W. Tang and F. Zhang, J. Am. Chem. Soc., 2019, 102, 4632 CAS.
- W. Song, X. Chen, L. Teng, Z. Zheng, J. Wen, F. Hu, R. Wei, L. Chen and H. Guo, J. Am. Chem. Soc., 2019, 102, 1822 CAS.
- R. Wei, L. Wang, F. Hu, X. Li, X. Peng, Y. Shi, H. Guo and J. Qiu, J. Lumin., 2018, 197, 291 CrossRef CAS.
- P. Yang, X. Yu, X. Xu, T. Jiang, H. Yu, D. Zhou, Z. Yang, Z. Song and J. Qiu, J. Solid State Chem., 2013, 202, 143 CrossRef CAS.
- E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274 CrossRef CAS.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J.-S. Lee and W. B. Im, Nat. Mater., 2017, 16, 543 CrossRef CAS PubMed.
- T. Komukai, Y. Sato, H. Kato and M. Kakihana, J. Lumin., 2017, 181, 211 CrossRef CAS.
- B. Li, X. Huang, H. Guo and Y. Zeng, Dyes Pigm., 2018, 150, 67 CrossRef CAS.
- H. Zhu, C. C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R.-S. Liu and X. Chen, Nat. Commun., 2014, 5, 4312 CrossRef CAS.
- Y. Chen, F. Pan, M. Wang, X. Zhang, J. Wang, M. Wu and C. Wang, J. Mater. Chem. C, 2016, 4, 2367 RSC.
- M. Zhao, H. Liao, M. S. Molokeev, Y. Zhou, Q. Zhang, Q. Liu and Z. Xia, Light: Sci. Appl., 2019, 8, 38 CrossRef.
- T.-T. Xuan, J.-Q. Liu, R.-J. Xie, H.-L. Li and Z. Sun, Chem. Mater., 2015, 27, 1187 CrossRef CAS.
- R.-J. Xie, N. Hirosaki, T. Suehiro, F.-F. Xu and M. Mitomo, Chem. Mater., 2006, 18, 5578 CrossRef CAS.
- X. Zhang, J. Yu, J. Wang, B. Lei, Y. Liu, Y. Cho, R.-J. Xie, H.-W. Zhang, Y. Li, Z. Tian, Y. Li and Q. Su, ACS Photonics, 2017, 4, 986 CrossRef CAS.
- L. Lv, X. Jiang, S. Huang, X. a. Chen and Y. Pan, J. Mater. Chem. C, 2014, 2, 3879 RSC.
- Z. Zhang, O. M. ten Kate, A. C. A. Delsing, Z. Man, R. Xie, Y. Shen, M. J. H. Stevens, P. H. L. Notten, P. Dorenbos, J. Zhao and H. T. Hintzen, J. Mater. Chem. C, 2013, 1, 7856 RSC.
- J. Ding, T. Seto, Y. Wang, Y. Cao, H. Li and Y. Wang, Chem. – Asian J., 2018, 13, 2649 CrossRef CAS.
- M. Shang, J. Wang, J. Fan, H. Lian, Y. Zhang and J. Lin, J. Mater. Chem. C, 2015, 3, 9306 RSC.
- Q. Zhao, G. Jiang, Z. Wang, M. Shi, B. Yang, J. Zou and T. Tu, J. Mater. Sci.: Mater. Electron., 2018, 29, 4011 CrossRef CAS.
- H. S. Kim, K.-i. Machida, T. Horikawa and H. Hanzawa, J. Alloys Compd., 2015, 633, 97 CrossRef CAS.
- J. H. Oh, H. Kang, Y. J. Eo, H. K. Park and Y. R. Do, J. Mater. Chem. C, 2015, 3, 607 RSC.
- E. Song, W. Zhao, G. Zhou, X. Dou, C. Yi and M. Zhou, J. Rare Earths, 2011, 29, 440 CrossRef CAS.
- H. Ji, Z. Huang, Z. Xia, M. S. Molokeev, V. V. Atuchin, M. Fang and S. Huang, Inorg. Chem., 2014, 53, 5129 CrossRef CAS PubMed.
- G. Zhu, Z. Ci, Y. Shi, M. Que, Q. Wang and Y. Wang, J. Mater. Chem. C, 2013, 1, 5960 RSC.
- W. Tang and Z. Zhang, J. Mater. Chem. C, 2015, 3, 5339 RSC.
- L. Jiang, R. Pang, D. Li, W. Sun, Y. Jia, H. Li, J. Fu, C. Li and S. Zhang, Dalton Trans., 2015, 44, 17241 RSC.
- C.-H. Huang, W.-R. Liu and T.-M. Chen, J. Phys. Chem. C, 2010, 114, 18698 CrossRef CAS.
- S. Liang, P. Dang, G. Li, M. S. Molokeev, Y. Wei, H. Lian, M. Shang, A. A. Al Kheraiff and J. Lin, J. Mater. Chem. C, 2018, 6, 6714 RSC.
- W. Tang, Z. Zhang, Y. Ma and D. Qin, Ceram. Int., 2017, 43, 9117 CrossRef CAS.
- C. Ding and W. Tang, Opt. Mater., 2018, 76, 56 CrossRef CAS.
- D. Wen, Z. Dong, J. Shi, M. Gong and M. Wu, ECS J. Solid State Sci. Technol., 2013, 2, R178 CrossRef CAS.
- M. Lu, C. Zhu, Z. Chen and M. Shi, Polyhedron, 2018, 153, 139 CrossRef CAS.
- R. Mi, Y.-g. Liu, L. Mei, C. Zhao, J. Chen, Z. Huang and M. Fang, J. Am. Ceram. Soc., 2017, 100, 3050 CrossRef CAS.
- J. Long, Y. Wang, C. Ma, X. Yuan, W. Dong, R. Ma, Z. Wen, M. Du and Y. Cao, RSC Adv., 2017, 7, 19223 RSC.
- J. Yan, Z. Zhang, D. Wen, J. Zhou, Y. Xu, J. Li, C.-G. Ma, J. Shi and M. Wu, J. Mater. Chem. C, 2019, 7, 8374 RSC.
- Z. Zhang, C. Ma, R. Gautier, M. S. Molokeev, Q. Liu and Z. Xia, Adv. Funct. Mater., 2018, 28, 41 Search PubMed.
- Z. Xia and K. R. Poeppelmeier, Acc. Chem. Res., 2017, 50, 1222–1230 CrossRef CAS.
- J. Qiao, L. Ning, M. S. Molokeev, Y.-C. Chuang, Q. Liu and Z. Xia, J. Am. Chem. Soc., 2018, 140, 9730–9736 CrossRef CAS PubMed.
- A. C. Larson and R. B. Van Dreele, Los Alamos National Laboratory Report LAUR 86, Los Alamos National Laboratory, Los Alamos, NM, 1994 Search PubMed.
- M. Yashima, A. Sakai, T. Kamiyama and A. Hoshikawa, J. Solid State Chem., 2003, 175, 272 CrossRef CAS.
- W.-R. Liu, C.-H. Huang, C.-W. Yeh, J.-C. Tsai, Y.-C. Chiu, Y.-T. Yeh and R.-S. Liu, Inorg. Chem., 2012, 51, 9636 CrossRef CAS PubMed.
- W. B. Park, H. Kim, H. Park, C. Yoon and K. S. Sohn, Inorg. Chem., 2016, 55, 2534 CrossRef CAS PubMed.
- S. R. S. Kumar, M. N. Hedhili, H. N. Alshareef and S. Kasiviswanathan, Appl. Phys. Lett., 2010, 97, 11 Search PubMed.
- Y. Wei, J. Gao, G. Xing, G. Li, P. Dang, S. Liang, Y. S. Huang, C. C. Lin, T.-S. Chan and J. Lin, Inorg. Chem., 2019, 58, 6376 CrossRef CAS PubMed.
- C.-H. Huang, Y.-C. Chen, T.-M. Chen, T.-S. Chan and H.-S. Sheu, J. Mater. Chem., 2011, 21, 5645 RSC.
- Q. Zhang, X. Wang, X. Ding and Y. Wang, Dyes Pigm., 2018, 149, 268 CrossRef CAS.
- Z. Xia, Y. Zhang, M. S. Molokeev and V. V. Atuchin, J. Phys. Chem. C, 2013, 117, 20847 CrossRef CAS.
- L. G. Vanquickenborne, P. Hoet and K. Pierloot, Inorg. Chem., 1986, 25, 4228 CrossRef CAS.
- N. Guo, Y. Huang, H. You, M. Yang, Y. Song, K. Liu and Y. Zheng, Inorg. Chem., 2010, 49, 10907 CrossRef CAS PubMed.
- X. Ding, G. Zhu, Q. Wang and Y. Wang, RSC Adv., 2015, 5, 30001 RSC.
- L. Shi, Y. Huang and H. J. Seo, J. Phys. Chem. A, 2010, 114, 6927 CrossRef CAS PubMed.
- Y. Zhang, Z. Wu, D. Geng, X. Kang, M. Shang, X. Li, H. Lian, Z. Cheng and J. Lin, Adv. Funct. Mater., 2014, 24, 6581 CrossRef CAS.
- M. Jiao, Q. Xu, C. Yang and M. Liu, J. Mater. Chem. C, 2018, 6, 4435 RSC.
- X. Zhang, Z. Zhu, Z. Guo, Z. Sun, Z. Yang, T. Zhang, J. Zhang, Z.-C. Wu and Z. Wang, Inorg. Chem. Front., 2019, 6, 1289 RSC.
- W. Sun, Y. Jia, R. Pang, H. Li, T. Ma, D. Li, J. Fu, S. Zhang, L. Jiang and C. Li, ACS Appl. Mater. Interfaces, 2015, 7, 25219 CrossRef CAS PubMed.
- P. Strobel, C. Maak, V. Weiler, P. J. Schmidt and W. Schnick, Angew. Chem., Int. Ed., 2018, 57, 8739 CrossRef CAS PubMed.
- C. Maak, D. Durach, C. Martiny, P. J. Schmidt and W. Schnick, Chem. Mater., 2018, 30, 3552 CrossRef CAS.
- M. Zhao, H. Liao, L. Ning, Q. Zhang, Q. Liu and Z. Xia, Adv. Mater., 2018, 30, 38 Search PubMed.
- A. Manoogian and J. C. Woolley, Can. J. Phys., 1984, 62, 285 CrossRef CAS.
- N. M. Ravindra and V. K. Srivastava, J. Phys. Chem. Solids, 1979, 40, 791 CrossRef CAS.
- C. Qin, Y. Huang, L. Shi, G. Chen, X. Qiao and H. J. Seo, J. Phys. D: Appl. Phys., 2009, 42, 18 Search PubMed.
- J. Wang, J. H. Hao and P. A. Tanner, J. Lumin., 2015, 164, 116 CrossRef CAS.
- F. Du, R. Zhu, Y. Huang, Y. Tao and H. J. Seo, Dalton Trans., 2011, 40, 11433 RSC.
- K. J. Laidler, J. Chem. Educ., 1984, 61, 494 CrossRef CAS.
- J. Y. Park, S. J. Park, B. K. Moon, M. Kwak, K. Jang and H. K. Yang, Chem. Phys. Lett., 2018, 708, 66 CrossRef CAS.
- G. Li, C. C. Lin, W.-T. Chen, M. S. Molokeev, V. V. Atuchin, C.-Y. Chiang, W. Zhou, C.-W. Wang, W.-H. Li, H.-S. Sheu, T.-S. Chan, C. Ma and R.-S. Liu, Chem. Mater., 2014, 26, 2991 CrossRef CAS.
- R.-J. Xie, N. Hirosaki, X.-J. Liu, T. Takeda and H.-L. Li, Appl. Phys. Lett., 2008, 92, 201905 CrossRef.
- L. Sun, B. Devakumar, J. Liang, S. Wang, Q. Sun and X. Huang, J. Alloys Compd., 2019, 785, 312 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The refined residual factors, cell parameters, fractional atomic coordinates, the concentration-dependent luminescence intensity of CMNPO:xZn2+ and CMNPO:yMg2+ phosphors. See DOI: 10.1039/c9tc05768d |
| This journal is © The Royal Society of Chemistry 2020 |