Low surface energy interface-derived low-temperature recrystallization behavior of organic thin films for boosting carrier mobility†
Abstract
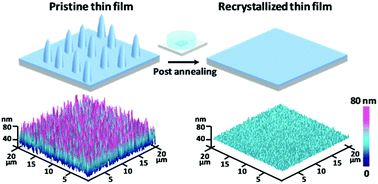
Due to low-temperature processing properties and high carrier mobilities, solution-processed small molecule organic thin-film transistors (OTFTs) are promising candidates for enabling low-cost flexible electronic circuits and displays. Much progress has been made in terms of material performance, however, there remain significant concerns about well understanding and controlling the morphology and polymorphism of soluble small molecule organic semiconductor thin films. Here, we investigated the physical mechanisms of the dielectric interfacial properties on the recrystallization of soluble small molecule organic semiconductor thin films, and demonstrated an effective route to improve the thin-film morphology and device performance. We found that a low surface energy interface can derive low-temperature recrystallization behavior of solution-processed organic semiconductor thin films, which has an important impact on boosting the carrier mobility of OTFTs. After low-temperature recrystallization, the carrier mobility of OTFTs is dramatically enhanced by about one order of magnitude. These attractive results suggest that this work allows a better understanding about the role of dielectric interfacial properties in assisting the thin-film recrystallization towards the desirable thin-film morphology, indicating promising potential for future low-cost high-performance solution-processed organic electronic devices.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...