Open Access Article
Open Access ArticleUnravelling the photothermal and photomechanical contributions to actuation of azobenzene-doped liquid crystal polymers in air and water†
Marina
Pilz da Cunha
 ,
Evelien A. J.
van Thoor
,
Evelien A. J.
van Thoor
 ,
Michael G.
Debije
,
Michael G.
Debije
 ,
Dirk J.
Broer
,
Dirk J.
Broer
 and
Albert P. H. J.
Schenning
and
Albert P. H. J.
Schenning
 *
*
Laboratory of Stimuli-Responsive Functional Materials & Devices, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology, P. O. Box 513, 5600 MB, Eindhoven, The Netherlands. E-mail: a.p.h.j.schenning@tue.nl
First published on 14th October 2019
Abstract
Liquid crystal networks doped with azobenzene molecular photo-switches have been commonly used as responsive polymers in untethered soft/micro robots. Understanding the underlying actuation mechanism of azobenzene chromophores in aqueous and ambient environments is crucial for the control of deformation speed and amplitude of the photo responsive actuators. Here, a systematic study is presented that clarifies the outstanding uncertainty in the mechanistic contributions of azobenzene powered actuation. The photothermal and photomechanical contributions in the mechanism are elucidated and the mechanistic dependence on azobenzene chemistry is demonstrated. By connecting mechanical, thermal, isomerization and actuation analysis in both dry and aqueous environments, this study reveals design guidelines for light-responsive amphibious soft robots.
Introduction
Over the last decade there has been an increasing interest in stimuli-responsive materials for a multitude of applications including smart textiles,1 drug delivery,2 microfluidics3 and soft robotics.4–6 Of particular interest are soft materials presenting mechanical deformation as a response to remotely addressed stimuli, including light,7,8 magnetic fields,9 humidity,10,11 and pH.12 As an untethered stimulus, light is commonly employed due to ease of application, high degree of tunability and temporal resolution. Liquid crystal networks (LCNs) are a prevalent class of soft smart materials, capable of functioning in dry environments with directed molecular self-assembly allowing for tuneable and complex nano-scale molecular designs. A prominent choice of photo-switch in light responsive LCNs are azobenzene derivatives due to their facile incorporation into the liquid crystalline network without disruption of molecular alignments.13 Photo responsivity is achieved through the reversible isomerization of the rod-like, stable trans azobenzene isomer into the bent cis isomer, accompanied by a shift in absorbance. LCNs containing azobenzene photo-switches have been designed to perform remotely controlled bending,14 curling15 and more complex oscillatory motion activated by light.16–18 Transforming light into mechanical motion has led to the development of LCN-based soft robots displaying inchworm-like walking,19,20 rolling21–23 and caterpillar-like24,25 locomotion in dry environments and a recent endeavour is to expand the locomotion into water-based environments through the development of micro-swimmers.26,27Elucidation of the complex mechanisms for photoinduced mechanical deformations observed in azobenzene-based LCN cantilevers has been extensively pursued in the literature, but an expanding library of azobenzene derivatives has left large uncertainties in uncovering mechanistic guidelines as to which azobenzene moiety to select for specific applications. Current literature suggests that the light-triggered actuation of LCNs containing azobenzene chromophores is rooted in both photomechanical and photothermal effects. Photomechanical28 factors are derived from isomerization-induced effects which include molecular order disruption29,30 and free-volume formation31,32 as a result of the cis isomer bent conformation. Additionally, the diminished molecular length of the azobenzene moiety from 9 to 5.5 Å,33 corresponding to trans and cis isomers respectively, can generate an effective isomer-generated network stress (pull-effects).34 Photothermal effects include isomerization-driven heating and network photo-softening,35–37 which are responsible for the large light-induced storage modulus decrease that significantly surpasses the commonly observed thermal-softening of glassy polymers.38 Understanding the interplay of molecular processes, mechanical and actuating properties will be crucial in advancing the field of soft robotics. An ambiguity remains in understanding of how the mechanism for photoinduced deformation differs between photo-switches of different chemical natures. Additionally, the molecular requirements for a photo-switch to induce actuation in both dry and aqueous environments is still largely concealed. Creation of such guidelines will be of great importance for the development of amphibious-like actuators. To date, no study has connected isomerization kinetic data, mechano-thermal tests with actuation amplitudes and rates to investigate which photomechanical or photothermal effects become dominant in the deformation mechanism of photo-switches of differing chemical natures in either air or aqueous environments.
We present a systematic study highlighting the nature of the azobenzene photo-switch and how it impacts the actuation mechanism. The networks investigated are splay aligned, containing low (2 mol%) concentrations of different photo-active azobenzene derivatives. Actuation is achieved through illumination using a collimated light source, leading the polymer films to undergo an uncurling motion directed towards the light source, Fig. 1A. Our choice of azobenzene moieties include two of the most popularly used derivatives for actuator fabrication, photo-switch DY and MR, Fig. 1C. We explore the effect of fully and partially attaching the chromophore to the network by comparing diacrylate and monoacrylate azobenzene moieties, as well as study the effect of cis isomer lifetime on actuation. Furthermore, this study is expanded to include actuation in aqueous environments. For the first time, we show a combined study that connects mechanical, thermal and actuation analysis with isomerization kinetic studies to uncover the relative contributions of photothermal and photomechanical effects to macroscopic bending in splay aligned liquid crystal cantilevers. This study provides essential guidelines for future designs of light responsive actuators by aiding the choice of azobenzene derivative most appropriate for each application.
Results and discussion
Preparation of the liquid crystal networks
The photo-responsive acrylate-based LCNs are composed of two liquid crystal mesogens (1 and 2, Fig. 1B) and 2 mol% of an azobenzene derivative. The azobenzene naming is based on the crosslink nature of the molecule: mono- (M) or di- (D) acrylate and on the color of the film, either yellow (Y) or red (R). The azobenzenes used were MY (monoacrylate azobenzene with a long cis lifetime (hours) see Fig. S1 (ESI†): the films have a yellow color), DY (diacrylate azobenzene with long cis lifetime (hours): films have has a yellow color) or MR (monoacrylate azobenzene with short cis lifetime: films have a red color),39Fig. 1C. Crosslink density is equal in all mixtures, adjusted by altering the content of crosslinker 1 in the film containing diacrylate azobenzene (mixture compositions in Table S1, ESI†). The network is photo-polymerized in a 20 μm gap glass cell filled with the liquid crystal (LC) mixture by capillary action. The cell employs homeotropic and planar alignment layers on either face of the cell for the realization of an overall splay alignment within the film. Polymerization is carried out at 80 °C in the nematic LC phase (see Fig. S2, ESI†). A subsequent thermal post-cure at 120 °C ensures full polymerization and releases mechanical stresses generated from polymerization shrinkage. For photo-polymerization of films containing azobenzene derivatives MY and DY, a filter was used to block wavelengths below 400 nm, preventing excessive trans–cis isomerization during polymerization.After opening the glass cells, the polymer films are cut into 5 × 20 mm2 strips, with the molecular director of the LC molecules parallel to the strip length. At room temperature, the splay films have a pre-curled shape, with the homeotropic side of the splay on the inside of the curl. The pre-curl is due to an increased molecular order at lower temperatures: cooling from the elevated polymerization temperature (80 °C) causes anisotropic molecular volume contraction and expansion, parallel and perpendicular to the molecular director, respectively (Fig. S3, ESI†). The splay aligned networks all show storage moduli (direction parallel to the planar aligned molecules) in the range of 1.2–1.8 GPa and glass transition temperatures (Tg) around 80 °C.
LCNs doped with azo-MR show a single intense absorption peak centered around 450 nm, Fig. 1D. The absence of a secondary peak corresponding to a cis absorption band is due to the derivative's very short cis lifetime, a result of the effective electron push–pull configuration generated by the ring substituted electron donating and accepting groups on opposing sides of the aromatic system. The other two azobenzene moieties, MY and DY, show equivalent extinction coefficients, seen by equal absorbance profiles (Fig. 1), and have cis isomers with longer lifetimes, 4 and 1.5 h in the dark, respectively, Fig. S1 (ESI†). The shorter cis lifetime of DY in the dark is likely due to the crosslinker nature of the moiety, facilitating the return to the stable trans state by the overall network. The trans state absorbance of both DY and MY is dominated by the π–π* absorption peak centered around 365 nm and a lower n–π* absorption at around 450 nm. Isomer trans–cis conversion is visualized by a decrease in the absorption at 365 nm and an increase at 450 nm.40
Photo-responsive actuation
The extent of actuation of the LCNs is observed to be heavily dependent on the light intensity used for actuation. Additionally, at equal light intensities, films containing different azobenzene moieties show strikingly different deformation amplitudes. The actuation efficiency for the individual films is illustrated as the maximum film tip displacement at varied illumination intensities of 365 or 455 nm light, Fig. 3A. Splay films doped with chromophore DY clearly display superior actuation efficiency upon illumination with 365 nm light: exposure of the same film to 455 nm light results in a slightly lowered actuation efficiency. On the other hand, actuation of MY doped films shows nearly no wavelength dependence, with both 365 and 455 nm illumination resulting in similar displacements. All films show a temperature increase when illuminated, the increase being linearly correlated to the incident light energy, Fig. 3B. The temperature increases caused by illumination of DY and MY films are identical, with a maximum temperature of 65 °C at 455 nm, 310 mW cm−2. This correlation is likely due to the equivalent absorption spectra of the two chromophores (Fig. 1C). The temperature increase observed is connected to the percentage light energy absorbed by the chromophore. In this way, one could expect 365 nm to lead to a higher film temperature as the trans state shows large spectrum absorption of the 365 nm LED (reaching above 80% absorption, see Experimental section for details). However, upon 365 nm illumination, fast trans–cis conversion (Fig. S1, ESI†), results in a dominant cis population, which absorbs only 60% of incident light. This absorption is similar to the 50% absorbed by the trans state at 455 nm, leading to a near wavelength independence of energy absorbed. The higher film temperatures reached for MR azobenzene (73 °C at 310 mW cm−2) is a consequence of the dye's greater light absorbance, i.e. higher extinction coefficient (Fig. 1C). Plotting the amplitude of displacement of the MR film against film temperature also shows a linear relationship, Fig. 3C. This plot demonstrates the near equivalent behavior of MY and MR doped films, suggesting that thermal effects play the governing role in the actuation mechanism of monoacrylate azobenzene chromophores.
The speed of underwater actuation with 365 nm is considerably slower than for actuation in air with similar light intensities (Fig. 4C). Underwater, the cantilever takes around 30 seconds to reach maximum unbending deformation, whereas in air maximum uncurling is achieved in the first 2 seconds of illumination. Likewise, the amplitude of underwater deformation of DY-azo films is significantly smaller than in air and appears to only slightly increase at intensities beyond 40 mW cm−2, Fig. 4D. This behavior is remarkably different than actuation in-air, which appears to increase with incident light energies, reaching a maximum flattening geometry at around 150 mW cm−2.
Mechanical, isomerization and thermal analysis
Upon illumination, azo-doped LCNs experience alterations in photo-switch isomer population, film temperature and mechanical properties.36,37 To study the inter-relation of these factors during illumination of films in a dry environment, the films are first irradiated with 455 nm for 3 minutes to ensure near-equivalent trans state populations, which we denote as a relative population of 100% trans. Equivalently, we denote 0% trans as the resulting absorbance profile after 3 min high intensity 365 nm illumination. Fig. 5 displays the effect of illumination of the splay films doped with MY and DY azobenzene derivatives with both 365 and 455 nm, considering sample temperature, storage modulus and isomer population. The same analysis is conducted for the MR derivative with single wavelength LED (455 nm), Fig. S6 (ESI†). Fig. 5 illustrates that illumination with 365 nm light promotes trans–cis isomerization and a rapid decrease in the trans population is observed for both azo-moieties (Fig. 5A and B). The isomer population is observed to remain constant when the light is turned off, as both chromophores show relatively long-lived cis isomers, Fig. S1 (ESI†). Accompanying the light triggered trans–cis isomerization, we observe an immediate increase in the film's surface temperature. Even though a constant trans population is established within the first few seconds of illumination, the film's temperature remains constant during illumination, as both cis and trans states show absorbance at 455 and 365 nm (Fig. 1C, vide supra). Ceasing illumination results in rapid cooling of the films. These observations suggest that film heating is independent of the population ratio between cis and trans isomers.Connection between molecular processes and macroscopic properties is observed by tracking the storage modulus of the films during the illumination process. Upon illumination, the polymer films show a decrease in the storage modulus concurrent with the temperature increase; higher light intensities result in greater modulus decrease. The plasticization of the network by excitation of the photo-switches has been described as the photo-softening effect. The three azo-benzene derivatives investigated in this work show similar degrees of softening, with no obvious wavelength dependence (see Fig. S7, ESI†). For both mono- and diacrylate chromophores, the storage modulus recovery upon turning off the excitation light correlates to the rapid temperature decrease of the film and not to the much slower isomer population reversion. Thus, we disconnect the network's mechanical properties from specific isomer populations and relate the material softening to the isomerization kinetics via temperature evolution. These observations suggest that photo-thermal effects such as network softening through isomerization of the azobenzene moieties are present in both mono- and diacrylate photo-switches.
Mechanisms for light triggered actuation
The mechanism for light-triggered actuation of LCNs containing azobenzene chromophores has roots in both photothermal and photomechanical factors. The contribution from each effect appears to be dependent on the nature of the photo-switch, considering its connection to the network (crosslinker or as a side-chain) and the life-time of the cis isomer. In Fig. 6 we present a roadmap to assist the distinction of the dominant factors in the actuation of mono- and diacrylate chromophores in both dry and aqueous environments. Azobenzene chromophores attached to the network as side-chains with short lived cis isomers have been classified as fully photothermal-driven photo-switches, with the heat released by cis–trans isomerization fueling actuation.18,43 The photothermal nature of splay azo-MR films is accentuated by the lack of actuation underwater upon exposure to light, as submersion in water suppresses film heating.The lack of large in-water actuation of the other monoacrylate azobenzene moiety (MY), suggests its actuation mechanism is also dominantly thermally driven. The actuation of films doped with MY-azo show negligible wavelength dependency in its actuation, further supporting a photothermal mechanism showing that the manner in which light is converted to heat (either through absorption of 365 or 455 nm light) has little impact. The reduced actuation in air for MY when compared to MR is due to its reduced light absorption: less energy is absorbed in the system resulting in lower film temperatures for comparable incident energies (Fig. 3B, vide supra). The negligible underwater deformation of films containing side-chain chromophores (Fig. S5, ESI†) suggests that the molecular disorder brought by the cis isomer configuration is not sufficient to generate large macroscopic actuation.
The remarkable underwater actuation of films doped with DY azobenzene moieties (unbending with trans–cis promoting 365 nm light exposure, and subsequent reversal with cis–trans promoting 455 nm light exposure, seen in Fig. 4C), suggests that crosslinking the chromophore to the network results in a more complex actuation mechanism. We propose aqueous actuation to be fully controlled by photomechanical deformation derived from the cis isomer populations, which generate an exerted network stress (pull-effect): this also explains the negligible light intensity dependence of deformation of DY films underwater. Isomerization kinetics show that low 365 nm intensity irradiation (20 mW cm−2) already converts 80% of trans states into the cis configuration, with increasing light intensities showing only slightly higher trans–cis conversion, Fig. S8 (ESI†). By converting the abundant cis population into the trans isomer via exposure to 455 nm, the films return to their initial curled state.
The same DY-azo film exposed to light in air presents a significantly different picture, with greater actuation amplitudes upon exposure to higher light intensities. The possible explanation for this and for the greater actuation efficiency of diacrylate moieties (Fig. 3A), is that the in-air actuation mechanism for DY films is likely a constructive concert between photomechanical and photothermal contributions. Our observations of the higher in-air actuation efficiency for diacrylate azobenzene moieties is in accordance with previous studies on planar aligned films that observe higher photo-generated stresses from crosslinker azobenzene compared to side-chain moieties.34,44 In contrast to the MY chromophore with long cis lifetime, films doped with DY-azo show wavelength dependent behavior when actuated in-air, with 365 nm illumination showing higher actuation efficiency than 455 nm. We propose that this wavelength dependent actuation derives from photomechanical network stress (pull-effect) contributions in diacrylate chromophores. The contribution of network stress (pull-effect) is illustrated by the difference in the amplitude of actuation comparing 455 nm and 365 nm illumination in air and by the underwater actuation through 365 nm illumination.
However, network pull-effects are not the only contribution leading to higher deformation amplitudes in diacrylate chromophores; 455 nm illumination of DY films show larger deformations than the MY films with identical absorbance profile. This observation suggests that additional photomechanical contributions are present in crosslinked azobenzene moieties, and such contributions are manifested during both illumination modes (455 or 365 nm). We suggest that this additional contribution is the consequence of effective molecular oscillations of the crosslinked chromophore between the two isomer configurations during illumination.31,45 This oscillation can create dynamic free volume by continuous trans–cis and cis–trans excitation, as both illumination wavelengths are absorbed by both isomeric forms. We speculate that a macroscopic manifestation of these molecular oscillations is only observable in combination with a thermal component, as no macroscopic manifestation of this effect is seen when the films are submerged in water. The importance of temperature in this photomechanical event is further illustrated by the increasing difference in the deformation amplitudes of the DY and MY azos at increasing light intensities (Fig. 3A), suggesting the contribution of this oscillation effect is more pronounced when the films heat up. We conclude that the actuation derived from diacrylate azobenzene moieties is rooted in both photothermal and photomechanical effects. Wavelength dependence is initiated by the mechanistic contribution of network pull-effects, directly controlled by isomer population, whereas photomechanical contributions from constant molecular oscillation between cis and trans states is independent of isomer population and is directly connected to the system's temperature.
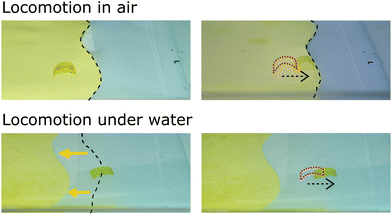
Amphibious walker
Elucidation of mechanistic contributions towards light actuation in azobenzene polymer films reveals guidelines for selecting the proper azobenzene chromophore for different applications. Monoacrylate azobenzene moieties show actuation dominated by photothermal mechanisms independent of isomer kinetics, making them excellent choices for dry environment applications with fast and reversible actuation in non-homogeneous aligned networks (such as splay) due to their relatively wavelength-independent behavior, fast reversibility and high actuation amplitude controlled through the regulation of the incident light intensity. On the other hand, applications targeted for aqueous or dual aqueous and non-aqueous application (using relatively low light intensities) will require the use of diacrylate chromophores, since actuation in water is dominated by photomechanical effects which are minimal in the monomeric azobenzenes. To illustrate the implications of our study we have created light-triggered locomotion of an inchworm-like soft robot (inspired by the design of Yamada et al.20) in both dry and wet environments. The actuator moves across a paper surface when illuminated from the top, Fig. 7. When the actuator contains DY azobenzene moiety, the soft robot is not only able to walk in a dry environment (Movie S1, ESI†), but when flooded with water (Movie S2, ESI†), the amphibious soft robot is still able to move while submerged (albeit at a slower speed than in air), Movie S3 (ESI†). Substitution of DY for monoacrylate azobenzene chromophore (MY), results in no locomotion underwater as the film shows no substantial underwater actuation.Conclusion
In this work, we have explored the actuation efficiency of splay aligned LCN films doped with three commonly used azobenzene chromophores in air and water. Through a combined mechanical and kinetic analysis, we have uncovered the governing factors in the actuation mechanism for chromophores depending on the photo-switch chemistry, setting guidelines for future applications. We demonstrated the higher actuation efficiency of crosslinker azobenzene moieties when compared to side-chain chromophores, due to a constructive contribution between photochemical and photothermal effects in the actuation mechanism. Regardless of cis isomer life-time, side-chain chromophores predominantly actuated due to photothermal contributions, highlighting that for amplified actuation, network anisotropy provided by splay or twisted nematic alignments is of great importance. Near-zero wavelength dependence for the actuation of side-chain azobenzene photo-switches with longer cis lifetimes has been demonstrated. For crosslinked azobenzene derivatives with relatively stable cis isomers, we observed that different illumination wavelengths “activate” certain actuation pathways in which either photochemical or photothermal effects dominate. This comprehensive study was used to fabricate a primitive amphibious soft robot walker and will provide essential design rules for the choice of azobenzene chromophores for applications in the field of light responsive actuators in air and water.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was made possible by a grant of Technology Foundation STW and the Impuls 2 program of the TU/e.References
- J. Hu, H. Meng, G. Li and S. I. Ibekwe, Smart Mater. Struct., 2012, 21, 1 Search PubMed.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2013, 65, 10–16 CrossRef CAS PubMed.
- J. A. Lv, Y. Liu, J. Wei, E. Chen, L. Qin and Y. Yu, Nature, 2016, 537, 179–184 CrossRef CAS PubMed.
- S. I. Rich, R. J. Wood and C. Majidi, Nat. Electron., 2018, 1, 102–112 CrossRef.
- H. Zeng, P. Wasylczyk, D. S. Wiersma and A. Priimagi, Adv. Mater., 2018, 30, 1–9 Search PubMed.
- L. Hines, K. Petersen, G. Z. Lum and M. Sitti, Adv. Mater., 2017, 29, 1–43 CrossRef PubMed.
- H. K. Bisoyi, A. M. Urbas and Q. Li, Adv. Opt. Mater., 2018, 6, 1800458 CrossRef.
- S. Nocentini, C. Parmeggiani, D. Martella and D. S. Wiersma, Adv. Opt. Mater., 2018, 6, 1–17 Search PubMed.
- J. Thévenot, H. Oliveira, O. Sandre and S. Lecommandoux, Chem. Soc. Rev., 2013, 42, 7099 RSC.
- R. C. P. Verpaalen, M. G. Debije, C. W. M. Bastiaansen, H. Halilović, T. A. P. Engels and A. P. H. J. Schenning, J. Mater. Chem. A, 2018, 6, 17724–17729 RSC.
- L. T. De Haan, J. M. N. Verjans, D. J. Broer, C. W. M. Bastiaansen and A. P. H. J. Schenning, J. Am. Chem. Soc., 2014, 136, 10585–10588 CrossRef CAS PubMed.
- G. Kocak, C. Tuncer and V. Bütün, Polym. Chem., 2017, 8, 144–176 RSC.
- A. Priimagi, A. Shimamura, M. Kondo, T. Hiraoka, S. Kubo, J. I. Mamiya, M. Kinoshita, T. Ikeda and A. Shishido, ACS Macro Lett., 2012, 1, 96–99 CrossRef CAS.
- G. N. Mol, K. D. Harris, C. W. M. Bastiaansen and D. J. Broer, Adv. Funct. Mater., 2005, 15, 1155–1159 CrossRef CAS.
- S. J. Aßhoff, F. Lancia, S. Iamsaard, B. Matt, T. Kudernac, S. P. Fletcher and N. Katsonis, Angew. Chem., Int. Ed., 2017, 56, 3261–3265 CrossRef PubMed.
- S. Serak, N. Tabiryan, R. Vergara, T. J. White, R. A. Vaia and T. J. Bunning, Soft Matter, 2010, 6, 779–783 RSC.
- N. Tabiryan, H. Koerner, K. M. Lee, T. J. White, T. J. Bunning, R. A. Vaia and M. L. Smith, Adv. Funct. Mater., 2011, 21, 2913–2918 CrossRef.
- A. H. Gelebart, G. Vantomme, E. W. Meijer and D. J. Broer, Adv. Mater., 2017, 29, 1–6 CrossRef PubMed.
- F. Ge, R. Yang, X. Tong, F. Camerel and Y. Zhao, Angew. Chem., Int. Ed., 2018, 57, 11758–11763 CrossRef CAS PubMed.
- M. Yamada, M. Kondo, R. Miyasato, Y. Naka, J. Mamiya, M. Kinoshita, A. Shishido, Y. Yu, C. J. Barrett and T. Ikeda, J. Mater. Chem., 2009, 19, 60–62 RSC.
- J. J. Wie, M. R. Shankar and T. J. White, Nat. Commun., 2016, 7, 1–8 Search PubMed.
- X. Lu, S. Guo, X. Tong, H. Xia and Y. Zhao, Adv. Mater., 2017, 29, 1–7 Search PubMed.
- M. Yamada, M. Kondo, J. I. Mamiya, Y. Yu, M. Kinoshita, C. J. Barrett and T. Ikeda, Angew. Chem., Int. Ed., 2008, 47, 4986–4988 CrossRef CAS PubMed.
- H. Zeng, O. M. Wani, P. Wasylczyk and A. Priimagi, Macromol. Rapid Commun., 2017, 39, 1–6 Search PubMed.
- M. Rogóż, H. Zeng, C. Xuan, D. S. Wiersma and P. Wasylczyk, Adv. Opt. Mater., 2016, 4, 1689–1694 CrossRef.
- S. Palagi, A. G. Mark, S. Y. Reigh, K. Melde, T. Qiu, H. Zeng, C. Parmeggiani, D. Martella, A. Sanchez-Castillo, N. Kapernaum, F. Giesselmann, D. S. Wiersma, E. Lauga and P. Fischer, Nat. Mater., 2016, 15, 647–653 CrossRef CAS PubMed.
- S. Ma, X. Li, S. Huang, J. Hu and H. Yu, Angew. Chem., Int. Ed., 2019, 2655–2659 CrossRef CAS PubMed.
- T. J. White, J. Polym. Sci., Part B: Polym. Phys., 2018, 56, 695–705 CrossRef CAS.
- K. D. Harris, R. Cuypers, P. Scheibe, C. L. van Oosten, C. W. M. Bastiaansen, J. Lub and D. J. Broer, J. Mater. Chem., 2005, 15, 5043 RSC.
- D. Liu, C. W. M. Bastiaansen, J. M. J. Den Toonder and D. J. Broer, Angew. Chem., Int. Ed., 2012, 51, 892–896 CrossRef CAS PubMed.
- D. Liu and D. J. Broer, Nat. Commun., 2015, 6, 1–7 Search PubMed.
- O. M. Tanchak and C. J. Barrett, Macromolecules, 2005, 38, 10566–10570 CrossRef CAS.
- G. S. Kumar and D. C. Neckers, Chem. Rev., 1989, 89, 1915–1925 CrossRef CAS.
- A. Sánchez-Ferrer and H. Finkelmann, Macromol. Rapid Commun., 2011, 32, 671–678 CrossRef PubMed.
- J. Vapaavuori, A. Laventure, C. G. Bazuin, O. Lebel and C. Pellerin, J. Am. Chem. Soc., 2015, 137, 13510–13517 CrossRef CAS PubMed.
- K. Kumar, A. P. H. J. Schenning, D. J. Broer and D. Liu, Soft Matter, 2016, 12, 3196–3201 RSC.
- J. M. Harrison, D. Goldbaum, T. C. Corkery, C. J. Barrett and R. R. Chromik, J. Mater. Chem. C, 2015, 3, 995–1003 RSC.
- G. J. Fang, J. E. MacLennan, Y. Yi, M. A. Glaser, M. Farrow, E. Korblova, D. M. Walba, T. E. Furtak and N. A. Clark, Nat. Commun., 2013, 4, 1–8 Search PubMed.
- A. H. Gelebart, D. Jan Mulder, M. Varga, A. Konya, G. Vantomme, E. W. Meijer, R. L. B. Selinger and D. J. Broer, Nature, 2017, 546, 632–636 CrossRef CAS PubMed.
- M. Poutanen, O. Ikkala and A. Priimagi, Macromolecules, 2016, 49, 4095–4101 CrossRef CAS.
- C. L. Van Oosten, K. D. Harris, C. W. M. Bastiaansen and D. J. Broer, Eur. Phys. J. E: Soft Matter Biol. Phys., 2007, 23, 329–336 CrossRef CAS PubMed.
- G. Vantomme, A. H. Gelebart, D. J. Broer and E. W. Meijer, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 1331–1336 CrossRef CAS PubMed.
- M. Pilz da Cunha, Y. Foelen, R. J. H. Van Raak, J. N. Murphy, A. P. Tom, M. G. Debije and A. P. H. J. Schenning, Adv. Opt. Mater., 2019, 1801643, 1–8 Search PubMed.
- M. Kondo, M. Sugimoto, M. Yamada, Y. Naka, J. I. Mamiya, M. Kinoshita, A. Shishido, Y. Yu and T. Ikeda, J. Mater. Chem., 2010, 20, 117–122 RSC.
- K. M. Lee and T. J. White, Macromolecules, 2012, 45, 7163–7170 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tc04440j |
| This journal is © The Royal Society of Chemistry 2019 |