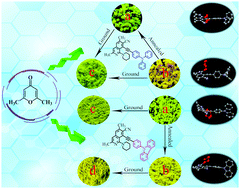
Two novel D–π–A isoquinoline derivatives IQ-TPA and IQ-PC using triphenylamine and 9-phenyl-9H-carbazole as an electron donor, respectively, were synthesized to investigate their applications in mechanofluorochromic (MFC) materials. The design was inspired by an accidentally obtained piperidine-1-substituted isoquinoline intermediate emitting strong blue solid-state fluorescence, which was obtained from the reaction of a 4H-pyran derivative with piperidine by a complex ring-opening/closing mechanism. These two compounds with highly twisted conformations both had two crystalline polymorphs in which the piperidin-1-yl group was located on different sides of the isoquinoline core. The different emissions of the polymorphs were confirmed to originate from their different molecular conformations. For IQ-TPA, yellow-emitting IQ-TPA-y could be converted into green-emitting IQ-TPA-g and exhibited hypsochromic MFC and thermochromic properties by the transformation between different crystalline states, respectively. Regarding IQ-PC, the two polymorphs did not exhibit MFC activities owing to the strong intermolecular interactions in the crystalline state, while the transition from green-emitting IQ-PC-g to yellow-emitting IQ-PC-y could be achieved by an annealing treatment. This indicated that different electron-donating groups led to different solid-state fluorescence stimulus-responsive properties. This work provides some useful information for the development of traditional fluorescent molecules with MFC, polymorphic, and thermochromic properties.