Weak ferromagnetic response in PbZr1−xTixO3 single crystals
Iwona
Lazar
 *a,
Monika
Oboz
a,
Jerzy
Kubacki
*a,
Monika
Oboz
a,
Jerzy
Kubacki
 a,
Andrzej
Majchrowski
b,
Julita
Piecha
a,
Dariusz
Kajewski
a,
Andrzej
Majchrowski
b,
Julita
Piecha
a,
Dariusz
Kajewski
 a and
Krystian
Roleder
a
a and
Krystian
Roleder
a
aInstitute of Physics, University of Silesia ul. 75 Pułku Piechoty 1, 41-500 Chorzów, Poland. E-mail: iwona.lazar@us.edu.pl
bInstitute of Applied Physics, Military University of Technology ul. Gen. Witolda Urbanowicza 2, 00-908 Warsaw, Poland
First published on 27th August 2019
Abstract
For the first time, a weak ferromagnetic hysteresis loop at room temperature has been observed in PbZr1−xTixO3 (PZT) single crystals. The occurrence of these properties has been related to the presence of oxygen vacancies and/or lower oxidation state of titanium. This proves that ferroelectric and ferromagnetic properties can occur simultaneously in this “famous” piezoelectric perovskite without any magnetic dopant.
The solid solutions of PbZr1−xTixO3 (PZT) are one of the most known piezoelectrics. Their large variety of applications have caused these materials to be intensely studied for decades. PZT ceramics are mainly used because of the difficulties to grow single crystals. Although there are some reports concerning their physical properties, there is still a deficiency of single crystals in the whole range of Ti content.1–6 Recently, we have been working on the technology of obtaining PZT crystals with homogeneous distribution of Ti ions in the crystal volume. We have finally succeeded in obtaining single crystals with x = 0.13, and their dielectric, elastic and piezoelectric properties have been reported in ref. 6 and 7. Additionally, the technology has also supplied defected crystals, and according to theoretical predictions8–10 such crystals may become very interesting from the point of view of appearance of magnetization in perovskite materials.
The literature on the magnetic properties of PZT solid solutions concerns mainly heterostructures,11–17 interfaces,18 ceramics,19 thin films20–23 or multilayers24,25 containing PZT, but there is no information on the ferromagnetic bulk properties of single PZT crystals. Moreover, what is important too, it concerns relatively high temperatures. Based on those theoretical predictions and the above experimental studies, we decided to check whether our defective PZT single crystals with average composition x = 0.10, with possible O and Ti vacancies and with the Ti3+ state, may also reveal magnetic properties, and this is the subject of this letter.
PZT single crystals were grown by a top-seeded solution growth technique. Magnetic measurements were performed using a Quantum Design MPMS-XL-7AC SQUID magnetometer. Magnetization isotherms were measured at 2 K, 10 K, 20 K, 40 K, 60 K, and 300 K in external fields up to 70 kOe. The electronic structure was examined by the X-ray photoelectron spectroscopy (XPS) method with the use of a PHI 5700/660 Physical Electronics spectrometer, using an Al Kα monochromatic X-ray source with an energy of 1486.6 eV. All the photoelectron spectra were calibrated against the peaks of Au 4f7/2 at 83.98 eV, Ag 3d5/2 at 368.27 eV, and Cu 2p3/2 at 932.67 eV of binding energy. The energy resolution in the XPS regime was about 0.35 eV. The test of the surfaces of the crystal was carried out at a standard take-off angle of 45°. The electronic structure of the PZT sample was tested at room temperature for the samples cleaved under UHV conditions. An electron float gun was used to compensate the surface positive charge appearing on the surface of insulating materials during X-ray irradiation. The core lines of O 1s, Zr 3d, Pb 4f, Ti 2p states and the valence band regions were recorded at pass energy 23.5 eV, and an energy step of 0.1 eV and 0.05 eV, respectively. Atomic concentration calculations were conducted from the analysis of the shape of the core lines, according to the standard procedure of Multipak (ver. 9.7.0.1). The deconvolution of photoemission lines was performed with a Doniach-Sunjic splitting function by means of the Simpeak program. The background was subtracted by the Shirley method.
Fig. 1 presents the dependence of magnetization M vs. magnetic field H for a single PZT crystal. In the low field range, i.e. below 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe for isotherms measured at 2 K, and below 16
000 Oe for isotherms measured at 2 K, and below 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe for isotherms measured at 10 K, 20 K, 40 K, 60 K and 300 K, ferromagnetic behaviour was observed. Above these H values diamagnetism was dominant. In the low magnetic field region, the M(H) curve at T = 300 K (Fig. 2) clearly shows a feature of ferromagnetic hysteresis with coercive field Hc = 65 Oe, and a remnant magnetization Mr = 1.1 × 10−3 emu g−1. Similar loops were observed for several different PZT crystals with the range of Hc = 65–100 Oe and Mr = (0.185–1.1) × 10−3 emu g−1.
000 Oe for isotherms measured at 10 K, 20 K, 40 K, 60 K and 300 K, ferromagnetic behaviour was observed. Above these H values diamagnetism was dominant. In the low magnetic field region, the M(H) curve at T = 300 K (Fig. 2) clearly shows a feature of ferromagnetic hysteresis with coercive field Hc = 65 Oe, and a remnant magnetization Mr = 1.1 × 10−3 emu g−1. Similar loops were observed for several different PZT crystals with the range of Hc = 65–100 Oe and Mr = (0.185–1.1) × 10−3 emu g−1.
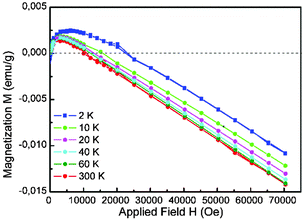 | ||
| Fig. 1 Magnetization M vs. magnetic field H at 2 K, 10 K, 20 K, 40 K, 60 K, and 300 K for a single PZT crystal. | ||
The temperature dependences of the magnetization under zero-field-cooled (ZFC) and field-cooled (FC) modes for the applied magnetic fields μ0H = 10 kOe and 30 kOe (Fig. 3) confirmed the isothermal measurements. The obtained room-temperature values of magnetic quantities are comparable to those for nanocrystalline PbTiO3 ceramics,19 but lower than those for e.g. PZT 52/48 thin films deposited on the alumina substrate,22 probably because of a greater amount of Ti and thus also Ti3+. To ensure that the presence of ferromagnetic properties in single PZT crystals is not a spurious effect, we have studied PbZrO3, which is one of the parent materials of these solid solutions. PbZrO3 has been chosen since it certainly contains defects in the Pb and O sublattices (it is difficult to avoid them during a technological process), but there are no Ti vacancies or the Ti3+ state. The magnetic measurements have shown that PbZrO3 is diamagnetic in the whole range of the applied magnetic field (inset of Fig. 2). Thus, we may conclude that the observed magnetism in the PZT crystals is not due to a spurious effect, and the role of titanium is crucial.
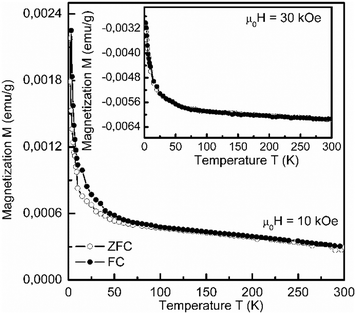 | ||
| Fig. 3 Temperature dependences of magnetization in zero-field-cooled (ZFC) and field-cooled (FC) modes for the PZT crystal. | ||
The main purpose of the XPS investigation was to check whether the Ti3+ state and oxygen vacancies are present, as well as magnetic contamination in the investigated PZT single crystals. The distribution of Pb, Ti, Zr, and O was examined, and huge differences in their content were noticed. However, the average concentration of the defected single PbZr1−xTixO3 crystal was estimated to be x = 0.10. It was also determined that in the crystal volume there were areas of more or less content of Pb. On the other hand, oxygen deficiency was found for all the studied areas. This means that the XPS atomic concentration analysis has evidently shown that the investigated single PZT crystal was not, as it was presupposed, stoichiometric. Moreover, the presence of different charge states of ions was detected. Due to the lack of oxygen ions in the crystal, changes in the valence states of Ti (from Ti4+ to Ti3+) and of Zr (from Zr4+ to Zr3+) were detected. As a consequence, the changes in the valence of Ti and Zr may contribute to the appearance of the Pb4+ state at the Pb2+ site. Furthermore, vacancies created at the Ti3+ position can be balanced by the occurred oxygen vacancies. Based on results reported26 all observed states can be described as:
In the analysis of the ionic states in the crystal, the dynamics of the process of filling the oxygen vacancies in the UHV chamber by residual gases under vacuum conditions has to be taken into account. Breaking the crystal under vacuum produces active centers with unsaturated bonds which, as a consequence, may bind these residual gases. We cannot exclude a diffusion within the crystal volume that plays a role in this process. However, we suppose that after breaking the crystal under vacuum (Fig. 4a) one can observe saturation of additional Ti 2p states localized at 457.3 eV (Fig. 4b) which can be ascribed to the Ti3+ electronic state. Finally, the re-creation of Ti3+ states is produced through reduction by means of Ar+ ion treatment (Fig. 4c). The intensity of the Ti3+ line decreases with time (as can be seen in Fig. 4a and b), which may point to the filling of oxygen vacancies and to the change from the Ti3+ to the Ti4+ state. A similar scenario which has been observed in the case of reduced SrTiO327 supports this explanation. The line within the Ti 2p multiplet, visible at about 457.3 eV, can thus be related to the Ti3+ species, which do not form metallic bonds of Ti.28
An oxygen vacancy is one of the most common and important point defects in perovskite oxides. The authors of a report29 showed that this defect causes redistribution of charge, relative displacements of ions and defect levels within the band gap, with respect to pure crystals. The influence of oxygen deficiency on the energy gap in the studied materials is shown in Fig. 5. In the case of the PZT crystal, a weak intensity of photoemission was detected, just below the Fermi level. In the parent materials PbZrO3 and PbTiO3 no photoemission was detected. A similar increase of photoemission in the energy gap, as a consequence of doping, was observed for Fe-doped SrTiO3.27 In the case of the SrTiO3 bulk crystals, the main contribution of titanium states to the valence band was observed in the range 3–8 eV,27,30,31 while the contribution in the 1–2 eV energy range was attributed to the Ti3+ state.32
According to the calculations in ref. 29, in the case of PbZrO3, the oxygen vacancy affects other atoms more than Zr. It is in contrast to PbTiO3, in which Ti is the most sensitive element. Based on that, we may assume that in the investigated crystal the oxygen defects affect the Ti ions first of all changing their valence or creating titanium vacancies. This may be the main reason why weak ferromagnetism has been detected in PZT, and not in PbZrO3. On the one hand, the calculated energies of surface defect formation point to an increase in the oxygen vacancy concentration at the surface compared to the bulk. On the other hand, the properties of oxygen vacancy depend on the chemical nature of the A and B atoms in ABO3 perovskites. That is why it could be the deep donor state in PbZrO3, but shallow in PbTiO3 and SrTiO3. The authors of ref. 29 have also reported that charge redistribution, relative displacements of ions, and defect levels within the band gap, caused by defect oxygen vacancies, have different values for the bulk and for the surface of oxide perovskites. Surface defects are more spatially delocalized and more energetically shallow than in the bulk, and thus the oxygen vacancy segregation towards the surfaces is possible.29 This predicted mechanism may explain the differences between the values of magnetic quantities obtained at room temperature for the investigated single PZT crystal and PZT thin films (see e.g.ref. 22).
In relation to all the results described above, one has to keep in mind that, according to theoretical prediction reported in ref. 8, titanium vacancies may generate ferromagnetism at high temperatures, i.e. calculations made for BaTiO3 showed that Ti vacancy, with a charge equal to −3, can survive even at 1300 K. Moreover, it was predicted that all vacancy species in BaTiO3 are able to induce ferromagnetism, and the magnitude of the optimal magnetic moment depends on their charge states. The highest values were estimated for the Ti defect with a charge equal to −3, −2, −1, or even 0, but that with the charge of −4 was not magnetic. Similarly, the dependence of the occurrence of magnetism on the charge state of oxygen vacancy was predicted; only neutral oxygen vacancy was ferromagnetic. All these defects may occur in a real material and if so there are strong interactions between them. The question remains when (i.e. at which concentration of those defects) these interactions are strong enough to generate the ferromagnetic response in the bulk perovskites. It is worth comparing that the magnetic moment of the single Ti3+ center, originating from oxygen vacancies, is estimated at about 0.05 μB,33 while the magnetic dopant of Fe maximally 2.2 μB per atom.33 This value is sufficient to create the multiferroic properties of perovskites by doping with iron [e.g.ref. 34]. Moreover, ferromagnetism at room temperature was observed in the bulk Nb-doped SrTiO3 single crystals, in which substitution of some Ti4+ ions by Nb+5 resulted in the Ti3+ state with unpaired electrons.33 As the defect-forming energy was estimated to have a lower value for bulk PbTiO3 and PbZrO3 than for bulk SrTiO3,29 this might explain why the Ti3+ state occurred without this type of substitution in our PZT. This showed that the concentration of defects inducing magnetism was sufficient to generate ferromagnetic properties in this single crystal without purposely introducing magnetic dopants. Therefore, the room-temperature ferromagnetism found in defected PZT crystals is comprehensible, especially due to the presence of the Ti3+ state and oxygen vacancies.
In summary, the results show that single PZT crystals “modified“ by defects created during the crystal growth process exhibit multiferroic behavior without doping with magnetic ions. This is the first report describing the magnetic properties of bulk PZT, and not as before, of various composites with solid solutions of lead titanate and lead zirconate. This makes PZT a potential multiferroic and makes it even more attractive from the point of view of application than ever before.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to sincerely thank Stanislav Kamba from the Czech Academy of Sciences in Prague for fruitful discussions on the magnetic properties of oxide perovskites. This work was supported by the National Science Centre, Poland [grant number 2016/21/B/ST3/02242].References
- J. Frantti, Y. Fujioka, A. Puretzky, Y. Xie, Z.-G. Ye and A. M. Glazer, J. Appl. Phys., 2013, 113, 174104 CrossRef.
- R. G. Burkovsky, Yu. A. Bronwald, A. V. Filimonov, A. I. Rudskoy, D. Chernyshov, A. Bosak, J. Hlinka, X. Long, Z.-G. Ye and S. B. Vakhrushev, Phys. Rev. Lett., 2012, 109, 097603 CrossRef CAS.
- D. Phelan, X. Long, Y. Xie, Z.-G. Ye, A. M. Glazer, H. Yokota, P. A. Thomas and P. M. Gehring, Phys. Rev. Lett., 2010, 105, 207601 CrossRef CAS.
- S. Gorfman, D. S. Keeble, A. M. Glazer, X. Long, Y. Xie, Z.-G. Ye, S. Collins and P. A. Thomas, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 020102 CrossRef.
- A. Bokov, X. Long and Z.-G. Ye, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 172103 CrossRef.
- I. Lazar, S. H. Oh, J.-H. Ko, P. Zajdel, D. Kajewski, A. Majchrowski, J. Piecha, J. Koperski, A. Soszyński and K. Roleder, J. Phys. D: Appl. Phys., 2019, 52, 115302 CrossRef.
- I. Lazar, D. Kajewski, A. Majchrowski, A. Soszyński, J. Koperski and K. Roleder, Ferroelectrics, 2016, 500, 67 CrossRef CAS.
- A. Raeliarijaona and H. Fu, Phys. Rev. B, 2017, 96, 144431 CrossRef.
- T. Shimada, Y. Uratani and T. Kitamura, Appl. Phys. Lett., 2012, 100, 162901 CrossRef.
- T. Xu, T. Shimada, Y. Araki, J. Wang and T. Kitamura, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 104106 CrossRef.
- J. Lian, F. Ponchel, N. Tiercelin, Y. Chen, D. Rémiens, T. Lasri, G. Wang, P. Pernod, W. Zhang and X. Dong, Appl. Phys. Lett., 2018, 112, 162904 CrossRef.
- H. Wu, S. D. Zhou, Y. Li, Y. G. Wang and F. M. Pan, Appl. Phys. Lett., 2018, 113, 102404 CrossRef.
- Y. C. Li, J. Wu, H.-Y. Pan, J. Wang, G.-H. Wang, J.-M. Liu and J.-G. Wan, Appl. Phys. Lett., 2018, 112, 212902 CrossRef.
- H. Su, J. Shen, H. Zhang, D. Wen, Y. Jing and X. Tang, J. Alloys Compd., 2016, 685, 546 CrossRef CAS.
- D. Mukherjee, M. Hordagoda, P. Lampen, M.-H. Phan, H. Srikanth, S. Witanachchi and P. Mukherjee, J. Appl. Phys., 2014, 115, 17D707 CrossRef.
- C. A. F. Vaz, J. Hoffman, Y. Segal, M. S. J. Marshall, J. W. Reiner, Z. Zhang, R. D. Grober, F. J. Walker and C. H. Ahn, J. Appl. Phys., 2011, 109, 07D905 CrossRef.
- C. A. F. Vaz, Y. Segal, J. Hoffman, R. D. Grober, F. J. Walker and C. H. Ahn, Appl. Phys. Lett., 2010, 97, 042506 CrossRef.
- H. Wu, S. D. Zhou, H. Ji, Y. Li and Y. G. Wang, J. Magn. Magn. Mater., 2019, 482, 25 CrossRef CAS.
- M. Wang, G.-L. Tan and Q. Zhang, J. Am. Ceram. Soc., 2010, 93, 2151 CrossRef CAS.
- V. R. Mudinepalli, P.-C. Chang, C.-C. Hsu, F.-Y. Lo, H.-W. Chang and W.-C. Lin, J. Magn. Magn. Mater., 2017, 432, 90 CrossRef CAS.
- W.-C. Lin, C.-W. Huang, Y.-C. Ting, F.-Y. Lo and M.-Y. Chern, J. Magn. Magn. Mater., 2015, 381, 446 CrossRef CAS.
- K. Sreelalitha and K. Thyagarajan, Mater. Res. Express, 2016, 3, 016403 CrossRef.
- A. Kumar, G. L. Sharma, R. S. Katiyar, R. Pirc, R. Blinc and J. F. Scott, J. Phys.: Condens. Matter, 2009, 21, 382204 CrossRef.
- G. Vats, A. Kumar, N. Ortega, Ch. R. Bowenf and R. S. Katiyar, Energy Environ. Sci., 2016, 9, 2383 RSC.
- Y. Zhang, Q. Zhou, J. Ding, Z. Yang, B. Zhu, X. Yang, S. Chen and J. Ou-Yang, J. Appl. Phys., 2015, 117, 124105 CrossRef.
- D. Damjanovic, Rep. Prog. Phys., 1998, 61, 1267 CrossRef CAS.
- J. Kubacki, D. Kajewski, J. Goraus, K. Szot, A. Koehl, Ch. Lenser, R. Dittmann and J. Szade, J. Chem. Phys., 2018, 148, 154702 CrossRef CAS.
- B. Psiuk, J. Szade, H. Schroeder, H. Haselier, M. Młynarczyk, R. Waser and K. Szot, Appl. Phys. A: Mater. Sci. Process., 2007, 89, 451 CrossRef CAS.
- Yu. F. Zhukovskii, E. A. Kotomin, S. Piskunov and D. E. Ellis, Solid State Commun., 2009, 149, 1359 CrossRef CAS.
- Y. Haruyama, S. Kodaira, Y. Aiura, H. Bando, Y. Nishihara, T. Maruyama, Y. Sakisaka and H. Kato, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 8032 CrossRef CAS.
- M. Takizawa, K. Maekawa, H. Wadati, T. Yoshida, A. Fujimori, H. Kumigashira and M. Oshima, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 113103 CrossRef.
- Y. Ishida, R. Eguchi, M. Matsunami, K. Horiba, M. Taguchi, A. Chainani, Y. Senba, H. Ohashi, H. Ohta and S. Shin, Phys. Rev. Lett., 2008, 100, 056401 CrossRef.
- Z. Q. Liu, W. M. Lü, S. L. Lim, X. P. Qiu, N. N. Bao, M. Motapothula, J. B. Yi, M. Yang, S. Dhar, T. Venkatesan and Ariando, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 220405 CrossRef.
- A. Kania, S. Miga, E. Talik, I. Gruszka, M. Szubka, M. Savinov, J. Prokleska and S. Kamba, J. Eur. Ceram. Soc., 2016, 36, 3369 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |




