Open Access Article
Open Access ArticleCobalt germanide precipitates indirectly improve the properties of thermoelectric germanium antimony tellurides†
Daniel
Souchay
a,
Stefan
Schwarzmüller
a,
Hanka
Becker
b,
Stefan
Kante
b,
G. Jeffrey
Snyder
 c,
Andreas
Leineweber
b and
Oliver
Oeckler
c,
Andreas
Leineweber
b and
Oliver
Oeckler
 *a
*a
aInstitute for Mineralogy, Crystallography and Materials Science, Faculty of Chemistry and Mineralogy, Leipzig University, Scharnhorststr. 20, 04275 Leipzig, Germany. E-mail: oliver.oeckler@gmx.de
bInstitute of Materials Science, TU Bergakademie Freiberg, Gustav-Zeuner-Str. 5, 09599 Freiberg, Germany
cDepartment of Materials Science and Engineering, Northwestern University, Evanston, IL 60208, USA
First published on 19th August 2019
Abstract
Different established synthesis methods such as melt-casting followed by annealing as well as melt-spinning or ball-milling followed by hot-pressing or spark plasma sintering may significantly influence the properties of thermoelectric materials. The comparison of microstructures obtained by different synthesis routes (water-quenching and melt-spinning followed by spark plasma sintering) reveals the indirect nature of the beneficial influence of cobalt germanide precipitates on the thermoelectric properties of germanium telluride and germanium antimony tellurides (GST materials). Cobalt germanide precipitates significantly influence the thermoelectric properties of GST materials: the thermoelectric figure of merit zT of (GeTe)17Sb2Te3 obtained by quenching melts in water increases from 1.6 to 1.9 (at 450 °C) by introducing cobalt germanide precipitates. They drastically reduce the grain and sub-grain sizes of the GST matrix. Melt-spinning followed by spark plasma sintering leads to nanoscopic cobalt germanide precipitates, whose effect on the thermoelectric properties, especially the phononic thermal conductivity, surprisingly seems to be marginal. This is due to the already significantly reduced (sub-)grain sizes in such polycrystalline GST samples as revealed by orientation (“channeling”) contrast in backscattered-electron micrographs. Melt-spun and spark-plasma-sintered GST material exhibits similar thermoelectric figures of merit zT values as water-quenched samples with cobalt germanide precipitates. In addition to providing easier access to samples with small (sub-)grains by simple quenching, the precipitates may stabilize the GST or GeTe microstructure by Zener pinning as cobalt is insoluble in the matrix material. This is very favorable concerning cyclability during thermoelectric measurements and potential applications. The heterostructured materials and their thermoelectric properties are long-term stable. Hall effect measurements of heterostructured GST (melt-spun and spark-plasma-sintered) indicate that the charge carrier concentration is near optimum.
Introduction
Germanium antimony tellurides (GeTe)nSb2Te3 (GST materials) have become well-known as the most prominent class of phase-change materials for non-volatile random access memory and optical data storage media.1–5 GST materials exhibit fast crystallization kinetics combined with high electrical conductivity and low thermal conductivity.6–8 The latter transport properties are also beneficial for thermoelectric materials.6Thermoelectric generators that convert waste heat to electrical energy may contribute to establish a multitude of sensors such as thermostatic radiator valves, transmitters, light sensors or aircraft monitoring,9,10 which require only a few hundred micro- or milliwatts to operate. Thus, only small temperature differences are necessary to make them autonomous as no cables or battery changes are necessary if they have access to waste heat.11 In addition, thermoelectric generators can provide independent energy supply in places where no other form of electricity is available, e.g. using thermoelectric generators in ventilators for stoves12 or in space missions.11 Thermoelectric materials may thus contribute to sustainable and renewable energy management, e.g. combining thermoelectric generators with salinity gradient solar pond, photovoltaic systems or by harvesting the waste heat of hot spring thermal energy.13,14
Many thermoelectric materials are being explored for such power generation applications, such as chalcogenides,15e.g. germanium telluride co-doped with BiTe and Cu with a zT value (see below) up to 1.55 in the temperature range of 325 to 450 °C,16 or PbTe co-doped by Na and Cl with Na on cation sites providing additional electrons to the conduction band, resulting in a zT value of 1.27 at 375 °C.17 Other recent examples include PbxSn1−xTe alloys, where Bi2Te3 doping increases zT in the low-temperature range (210 to 340 °C) compared to pristine PbxSn1−xTe samples,18 as well as silicides15 such as higher manganese silicides exhibiting textured behavior leading to ∼10% zT enhancement parallel to the pressing direction. Such anisotropic behavior in non-cubic polycrystalline thermoelectric materials should be carefully considered.19 Concerning the rather isotropic half-Heusler compounds, e.g. doping Ti0.3Zr0.35Hf0.35NiSn with Sb and/or Bi increases in zT value up to 0.58 at 550 °C.20
However, research for optimized thermoelectric materials faces several problems, especially their thermal stability and their rather low efficiency. The latter depends on the dimensionless figure of merit zT = S2σT/(κe + κph), where κe and κph are the electronic and phononic thermal conductivities, respectively, S the Seebeck coefficient, σ the electrical conductivity and T the temperature. In spite of the seemingly simple equation, the individual transport properties contributing to zT are highly correlated: σ depends on the charge carrier density n and the mobility μ according to σ = neμ (Drude model).21 High S would be beneficial for high zT values and requires a high effective mass m* according to S = (8π2kB2m*T) (π/3n)2/3 (3eh2)−1, assuming a parabolic band model with energy independent scattering approximation.22 However, a high m* would mean low μ according to μ = eτ/m* (τ = scattering relaxation time) which results in a low σ. High σ, on the other hand, results in high total thermal conductivity κ according to the Wiedemann–Franz law κeσ−1 = LT (L = Lorenz number).23 A decrease in L is correlated with an increase in thermopower, i.e. the absolute value of Seebeck coefficient according to L = 1.5 + e(−|S|/116) (where L is in 10−8 W Ω K−2 and S in μV K−1).24 This equation is a good approximation when a single parabolic band is applicable and when acoustic phonon scattering dominates. κph is one parameter which can be influenced by real-structure effects such as domain boundaries or nanoscale precipitates. However, the energy range of long-wavelength acoustic phonon modes has to be in the same range as the presumed phonon scattering centers as shown for e.g. skutterudites.25 The so-called phonon-glass electron-crystal (PGEC) concept26 focuses on disorder and “rattling” atoms that may enhance phonon scattering by reducing the mean free path of phonons, whereas σ ideally remains almost unaffected if electron or hole conduction is associated with the rigid framework.
The free path distribution range of phonons and thus the thermal conductivity may further be reduced by micro- or nanoscaled side phases in heterostructured systems.27 For example, such secondary phases in GST materials such as elemental germanium28 or antimony29 have been shown to increase zT significantly. Endotaxially intergrown nanoinclusions of SrTe in PbTe lead to peak zT values up to ∼2.2 at 640 °C.30 LAST (Pb–Sb–Ag–Te) thermoelectric materials obtained by quenching melts exhibit Ag–Sb-rich nanoscale inclusions with coherent or semicoherent interfaces in a PbTe-rich matrix; and further nanoscale inclusions related to compositional fluctuations of Ag, Sb and Pb/Sn have been described in the system Ag(Pb1−ySny)mSbTe2+m.31,32 Such decrease in lattice thermal conductivity has, however, also been explained by solid solution effects, e.g. for PbTe alloyed with MgTe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and strain effects in PbTe with nanoinclusions of SrTe.34
33 and strain effects in PbTe with nanoinclusions of SrTe.34
Instead of nano- and micro-inclusions of second phase, pronounced real structure effects in chemically homogeneous GST materials are also beneficial concerning their thermoelectric properties.6,35–39 Compounds (GeTe)nSb2Te3 with n ≥ 3 exhibit a disordered rocksalt-type high-temperature modification. Quenching this phase affords a metastable pseudocubic modification with short-range vacancy ordering and herringbone-like twinned nanostructures. The planar defects present reduce thermal conductivity drastically, most likely by scattering long-wavelength phonons.38,40 For n < ∼12, the stable room temperature (RT) phases correspond to long-periodically ordered layered structures that consist of rocksalt-type building blocks separated by van der Waals gaps. Increasing the GeTe content to n ≥ 12 leads to materials that can be considered as doped variants of GeTe. In the GST class of materials, many elements are suitable for substitution, e.g. Mn,41 Sn,42 Cd,43 Li,44,45 Ag,46 or Cr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 replacing Ge; As
47 replacing Ge; As![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 or In
48 or In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49,50 replacing Sb, and Se
49,50 replacing Sb, and Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 replacing Te. These substitutions change the electronic structure and charge carrier density and thus influence the thermoelectric performance.
35 replacing Te. These substitutions change the electronic structure and charge carrier density and thus influence the thermoelectric performance.
Comparable to GST composite materials (see above), some heterostructured variants of GeTe exhibit high zT values. Ge0.87Pb0.13Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 with zT = 1.9 at 500 °C and Ge0.87Pb0.13Te with 3 mol% Bi2Te3
51 with zT = 1.9 at 500 °C and Ge0.87Pb0.13Te with 3 mol% Bi2Te3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 (zT = 1.9 at 500 °C) both contain PbTe-rich domains as a side phase. The heterostructured material with the nominal composition Ge0.9Sb0.1Te0.9Se0.05S0.05
52 (zT = 1.9 at 500 °C) both contain PbTe-rich domains as a side phase. The heterostructured material with the nominal composition Ge0.9Sb0.1Te0.9Se0.05S0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 represents an Sb-doped GeTe1−xSex matrix containing GeS1−xSex precipitates. By substituting Te in GeTe with Se and S, κph was reduced by phonon scattering due to mass fluctuations. As an effect of spark plasma sintering, κph was further reduced by alloying with Sb, which leads to additional point defects and grain boundaries. However, these heterostructured samples combine substitution of the matrix material with additional precipitates, which makes it difficult to trace individual effects.
53 represents an Sb-doped GeTe1−xSex matrix containing GeS1−xSex precipitates. By substituting Te in GeTe with Se and S, κph was reduced by phonon scattering due to mass fluctuations. As an effect of spark plasma sintering, κph was further reduced by alloying with Sb, which leads to additional point defects and grain boundaries. However, these heterostructured samples combine substitution of the matrix material with additional precipitates, which makes it difficult to trace individual effects.
Furthermore, the microhardness of such precipitate-containing samples is increased compared to GeTe.53 In general, heterostructuring affects mechanical properties by maintaining the microstructure, which is well-known e.g. for δ-ferrite precipitates in steel.54 Grain-boundary migration can be impeded by small particles, an effect known as Zener pinning.55,56 Such particles generate a pinning pressure as it is energetically unfavorable to move past the particle because new boundaries must be created. This counteracts the driving force pushing the boundaries during annealing (mainly the energy stored by grain boundary curvature, especially at high-angle grain boundaries). This aspect is highly relevant for thermoelectric materials with respect to maintaining the microstructure and thus ensuring long-term performance.
In order to study the influence of precipitates in GST materials, a composition close to the optimal charge carrier concentration is most relevant. As precipitates should not influence the GST material by substitution, cobalt germanides are a particularly good choice as cobalt is almost insoluble in GeTe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 and GST with cobalt-rich precipitates.58,59 Thus, this is an optimal model system to analyze the effect of precipitates on thermoelectric properties. Comparing (GeTe)nSb2Te3 with different values of n with and without cobalt germanide precipitates is an intriguing way to elucidate the influence of precipitates on thermoelectric properties. Since good thermoelectric properties for GST with 1 wt% of CoGe2 precipitates had been demonstrated, these compositions served as basis for this study.58 Different synthesis routes such as quenching in water compared with melt-spinning (MS) followed by spark plasma sintering (SPS) allow for comparing more or less coarse microstructures.
57 and GST with cobalt-rich precipitates.58,59 Thus, this is an optimal model system to analyze the effect of precipitates on thermoelectric properties. Comparing (GeTe)nSb2Te3 with different values of n with and without cobalt germanide precipitates is an intriguing way to elucidate the influence of precipitates on thermoelectric properties. Since good thermoelectric properties for GST with 1 wt% of CoGe2 precipitates had been demonstrated, these compositions served as basis for this study.58 Different synthesis routes such as quenching in water compared with melt-spinning (MS) followed by spark plasma sintering (SPS) allow for comparing more or less coarse microstructures.
Experimental
Synthesis
Bulk samples were obtained by fusing ∼3 g (water-quenched samples) and ∼10 g (MS/SPS samples) of the elements Co (smart elements, 99.98%), Ge (Haines & Maassen 99.999%), Sb (VEB Spurenmetalle Freiberg, 99.9999%), and Te (Haines & Maassen 99.9%) in silica glass ampules under dry Ar atmosphere at 950 °C for at least 8 hours and quenching the melts at air (for MS/SPS) or in water (water-quenched). The water-quenched samples were annealed at 590 °C for 24 h, again followed by quenching in water. Ingots for MS and SPS were induction-melted in a custom-built melt-spinner using silica-glass tubes with a round nozzle measuring 1 mm in diameter. The tube and its surrounding were constantly flushed by inert Ar gas (Fig. S1, ESI†). Immediately after a sample was completely remolten, the melt was ejected onto a rotating copper wheel. The wheel speed, nozzle-wheel gap and ejection pressure were 1500 rpm, 0.3 mm and 0.35 bar (Ar overpressure), respectively. The obtained flakes (approximately 8 g) were placed in a 12 mm diameter graphite die and densified using an SPS apparatus (HDP25, FCT Systeme, Germany) by heating to 475 °C with 100 K min−1 and holding this temperature for 15 min with an uniaxial pressure of 70 MPa (more details given in Table S1, ESI†). Chemical compositions of all samples are listed in Table S2, ESI.† Disc-shaped specimens for thermal conductivity measurements and cuboid slabs of the same samples for measurements of the electrical resistivity and the Seebeck coefficient were sawn out from the SPS pellets or water-quenched ingots using a diamond wire saw and polished with SiC grinding powder until they were plane-parallel.Analytical methods
Electron microscopy
Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) were done using a Zeiss LEO 1530 Gemini instrument (acceleration voltage 20 kV) equipped with an EDX detector (INCA software,63 Oxford Instruments). Microstructural investigations were carried out using polished (final stage: colloidal silica suspension; OP-S, Struers) cross sections perpendicular to the disc plane of the samples using the orientation contrast (“channeling”) of backscattered electron (BSE) for imaging.Selected-area electron diffraction (SAED), high resolution transmission electron microscopy (HRTEM) and further EDX measurements were executed on a Philips CM-200 STEM (LaB6 cathode, 200 kV, super-twin lens, point resolution 0.23 nm) equipped with an RTEM 136-5 EDX detector (EDAX, Genesis64 software). A double-tilt low-background sample holder (Gatan) was used. For TEM investigations, the bulk material was manually cut, mechanically polished using a dimple grinder (Gatan) and then Ar-ion-thinned (Duo-Mill, GATAN). Evaluation of SAED data was done using the Analysis65 software. For HRTEM and SAED simulations, jEMS66 was used.
Transport properties
Thermal diffusivity was measured on samples with thicknesses of 0.7–1.75 mm under He atmosphere with a Linseis LFA 1000 apparatus equipped with an InSb detector. Simultaneous heat loss and finite pulse corrections were applied using Dusza's model.67 Values were averaged from five measurement points at each temperature. For the calculation of κ (= κe + κph), they were multiplied with the Dulong-Petit heat capacity and the density as derived by the weight and the volume determined by Archimedes’ principle with a precision of 0.03 g cm−3. Previous work has shown that the heat capacities of several GST materials28,35,38 agree well with the Dulong-Petit values. Details on heat capacities and densities (all >98.5% of the X-ray densities) are given in Table S2, ESI.†S and σ were measured simultaneously under He atmosphere with a Linseis LSR-3 instrument with NiCr/Ni and Ni contacts and a continuous reversal of the polarity of the thermocouples (bipolar setup). Measurements comprised three heating/cooling cycles between 50 °C and 500 °C with 25 °C steps at 10 K min−1 heating rate; a temperature gradient of 50 K was applied and three data points were acquired per temperature. All samples were polycrystalline (see Fig. 1 and Fig. S12, ESI†) with the dimensions of 1.51–2.02 mm × 1.55–2.5 mm × 7.21–8.84 mm; there is no evidence for pronounced preferred orientation. This is corroborated by the fact that no significant deviation in transport properties was detected when measuring vertical or horizontal to press direction of the SPS samples (Fig. S2, ESI†). The errors of S and σ are smaller than 10%; respectively for κ, they are approximately 5%. The combined absolute uncertainty of the measurements may amount up to 15% for the zT values. For further characterization, Hall measurements were carried out under dynamic vacuum applying a magnetic field of 2 T and currents of 100 mA. Using the van der Pauw technique, metal contacts were fixed onto the samples by screws.68Results and discussion
Phase composition and crystal structures
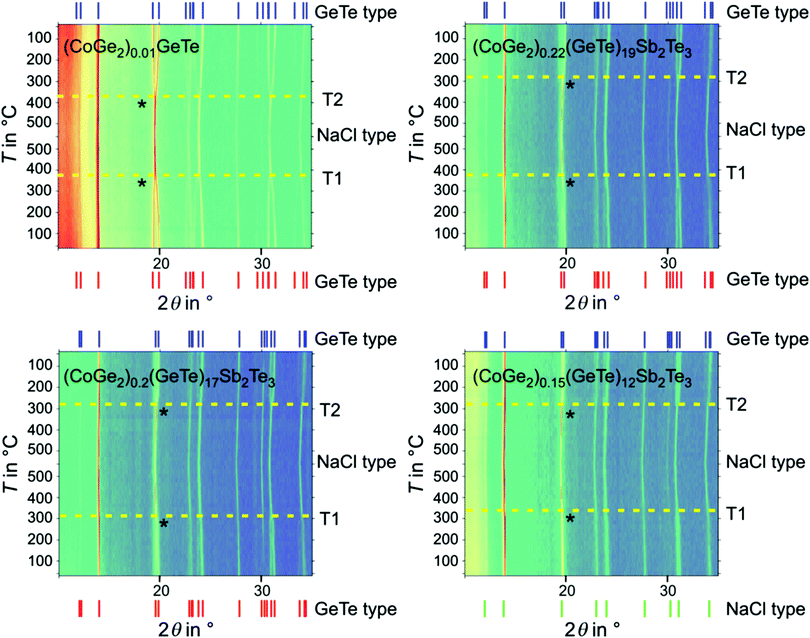
In order to evaluate the influence of the cobalt germanide precipitates on the thermoelectric properties, GeTe was compared with two corresponding heterostructured materials that formally contain 1 wt% CoGe2 or 1 wt% Co5Ge7 according to the nominal composition, assuming that Co is insoluble in the GST matrix. Samples obtained by MS and SPS were compared with water-quenched ones annealed 1 day at 590 °C. In the same manner, (GeTe)nSb2Te3 with n = 12, 17 and 19 and the corresponding heterostructured materials with formally 1 wt% CoGe2 were characterized (the weight fractions correspond to the nominal compositions of the starting materials and are represented as e.g. (CoGe2)x (matrix material) in the following). The values of the index x vary to ensure the same (at least formal) weight fraction of precipitates in all heterostructured materials.Samples with GeTe as the matrix material exhibit the α-GeTe structure type at RT, both after the initial synthesis and after additional thermal treatment during thermoelectric measurements. This holds for water-quenched as well as MS/SPS samples (Fig. S3 and S4, ESI†). Compounds (GeTe)nSb2Te3 (n = 12, 17 and 19), both pristine and as matrix materials, exhibit average structures that correspond to the rhombohedral α-GeTe type (Fig. S5, ESI†). The pseudocubic structure described for n = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 is not detected after MS preparation. This may be due to the small grain size in contrast to that in compact ingots, which enables relaxation to rhombohedral metrics in MS flakes. After densification by SPS, including rapid cooling from 450 °C, the structure of the (GeTe)12Sb2Te3 matrix corresponds to the metastable pseudocubic state (Fig. S6, ESI†), which is in good agreement with literature.38 GST samples with n = 17 and n = 19 retain the average rhombohedral α-GeTe-type structure. After thermal treatment during thermoelectric measurements, PXRD patterns of all samples investigated show this structure type, as indicated by broadened and/or split reflections (Fig. S6 and S7, ESI†). The likely presence of vacancy ordering, which eventually may lead to van der Waals gaps, cannot be evaluated on the basis of PXRD data, since the corresponding diffraction patterns are very similar (pseudo-homometry).70 Note that in the case of extended van der Waals gaps, the α-GeTe type only approximates the exact average structure of heavily disordered materials.
69 is not detected after MS preparation. This may be due to the small grain size in contrast to that in compact ingots, which enables relaxation to rhombohedral metrics in MS flakes. After densification by SPS, including rapid cooling from 450 °C, the structure of the (GeTe)12Sb2Te3 matrix corresponds to the metastable pseudocubic state (Fig. S6, ESI†), which is in good agreement with literature.38 GST samples with n = 17 and n = 19 retain the average rhombohedral α-GeTe-type structure. After thermal treatment during thermoelectric measurements, PXRD patterns of all samples investigated show this structure type, as indicated by broadened and/or split reflections (Fig. S6 and S7, ESI†). The likely presence of vacancy ordering, which eventually may lead to van der Waals gaps, cannot be evaluated on the basis of PXRD data, since the corresponding diffraction patterns are very similar (pseudo-homometry).70 Note that in the case of extended van der Waals gaps, the α-GeTe type only approximates the exact average structure of heavily disordered materials.
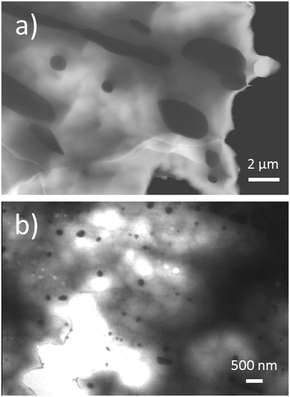
SEM-EDX measurements confirm that the composition of the different GST and GeTe matrices are in good agreement with the initial weights (Tables S3–S5, ESI†) and corresponding SEM images show heterostructured materials (example in Fig. 1). As it is typical for GeTe,71 small amounts of Ge precipitates were observed in some SEM images of some Sb-free samples. As weight fraction of precipitates is low, their structure could not be elucidated from PXRD patterns. TEM-EDX point measurements of the cobalt germanide precipitates, however, confirm compositions of approximately Co5Ge7 and CoGe2 (Fig. S8 and Table S6, ESI†). In addition to previously observed58 precipitates with Co5Ge7-type structure (space group I4mm, a ≈ 7.6 Å, c ≈ 5.8 Å),72 the structure and compositions of precipitates with the EDX analysis result Co30.5(1)Ge66.3(4)Sb0.3(1)Te3(2) correspond to CoGe2 (space group Cmce, a = 10.82 Å, b = 5.68 Å and c = 5.68 Å)73 according to electron diffraction. The corresponding SAED patterns and tilt angles between them are given in Fig. S9 and S10, ESI.† The pseudo-tetragonal metrics often leads to twinning, which was typically observed in SAED patterns. As both Co5Ge7 and CoGe2 precipitates are present in the heterostructured materials, the idealized stoichiometry of the precipitates slightly deviates from the nominal composition. The cobalt germanide precipitates may also contain very small amounts of Te, which most likely has no pronounced effect on their weight fraction. As GST materials exhibit a certain homogeneity range,36 this deviation is negligible as no other types of precipitates were detected. In the following sections, all heterostructured samples are represented by their formal nominal compositions in order to avoid the repetition of this discussion.
Microstructure
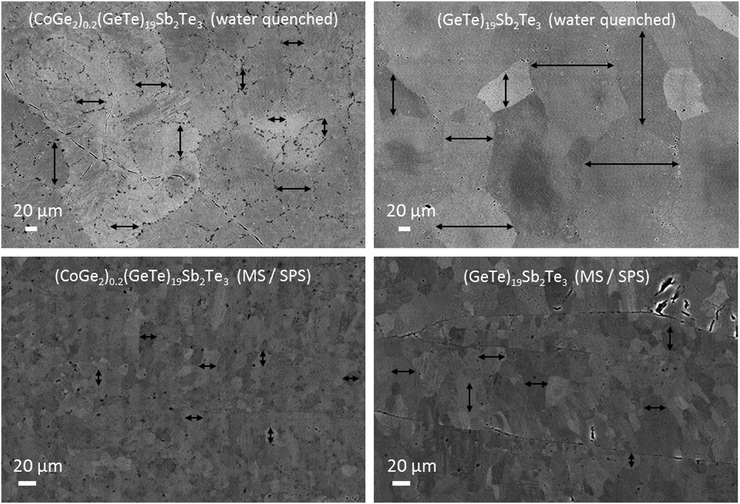
In order to understand how cobalt germanide precipitates in (GeTe)nSb2Te3 (n = 12, 17 und 19) influence thermoelectric properties, it proved important to compare the microstructure of the samples obtained by melting and water-quenching with the ones prepared by MS and subsequent SPS. In (CoGe2)0.2(GeTe)17Sb2Te3 and (CoGe2)0.22(GeTe)19Sb2Te3, the growth of the small grains and sub-grains of the as-cast GST matrix during thermal treatment is most likely hindered by the presence of cobalt germanide precipitates (Fig. 1 and Fig. S11, ESI†). Only these two materials have been studied in this regard, as they have the best average zT values among GST materials. Compared to MS/SPS samples, the relative effect of precipitates on the reduction of grain and sub-grain sizes is much more pronounced for water-quenched samples, where the higher-melting cobalt germanide precipitates might act as nucleation centers for the matrix material in addition to their main effect of preventing coarsening of the matrix’ microstructure. In samples obtained by MS/SPS, rapid quenching during MS outweighs the effect of the precipitates and leads to a very small grain size of the matrix material. In addition, MS samples contain much smaller cobalt germanide precipitates as compared to water-quenched samples (Fig. 2). These precipitates do not coarsen significantly upon thermal treatment during thermoelectric measurements; there are no precipitates >500 nm, which frequently occur in water-quenched samples (Fig. 1 and 2). This is explained by the fact that cobalt is not solved in the GST matrix according to EDX measurements (see Tables S3 and S5, ESI†), which impedes the growth of the precipitates by diffusion through GST. This seems reasonable as Co is insoluble in GeTe.57 Samples that contain cobalt germanide precipitates and exhibit reduced grain and/or subgrain sizes seem to be less brittle and to have higher toughness than single-phase ones. In addition to that, precipitates and small (sub-)grain sizes might also increase the hardness of heterostructured materials.53 Both in pristine and heterostructured, and both in water-quenched and in MS/SPS samples, grains of the GST matrix exhibit the typical “herringbone-like” nanodomain structures (Fig. S12, ESI†). Since these are present in all GST samples, the influence of the precipitates on the herringbone structure is negligible. These domain structures are well known from GeTe-related thermoelectric materials such as Ge0.9Sb0.08Ag0.02Te, Ge0.88Sb0.08Ag0.04Te and GST.40,58 The precipitates help to stabilize the grain size of the polycrystalline matrix material by Zener pinning;55,74 the microstructures do not change significantly during thermal cycling in physical measurements (Fig. S13, ESI†). As a result, GST samples with cobalt germanide precipitates are characterized by smaller grain sizes compared with the pristine GST materials after thermal treatment (Fig. 1).Temperature-dependent phase transitions
Temperature-dependent PXRD (Fig. 3 and Fig. S14, ESI†) reveals phase transitions in the GeTe and GST matrices of heterostructured samples after MS/SPS. The matrix materials GeTe, (GeTe)17Sb2Te3 and (GeTe)19Sb2Te3 show a reversible transition between the rhombohedral α-GeTe-type at low temperatures and the rocksalt structure type at HT, both during heating (T1; Fig. 3) and cooling (T2; Fig. 3).Quenched (GeTe)12Sb2Te3, however, exhibits a pseudocubic structure at RT, which transforms to a rocksalt-type high temperature structure (T1; bottom right in Fig. 3). Upon slow cooling, this sample also transforms to a distorted variant of rhombohedral α-GeTe type as average structure (T2; Fig. 3), which is in good agreement with literature.38,69
Phase transitions are visible because reflections of the rocksalt-type structure type split in a characteristic way. These phase transitions correlate with the hystereses in the measurements of thermoelectric properties (e.g. electrical conductivity, Fig. 4 and Table S7, ESI†). As the phase transition from GeTe type to rocksalt-type itself is a displacive phase transformation, these structure models represent average structures and the hystereses (Fig. 4) are caused by vacancy diffusion and element ordering during heating and cooling as reported in the literature.75–77
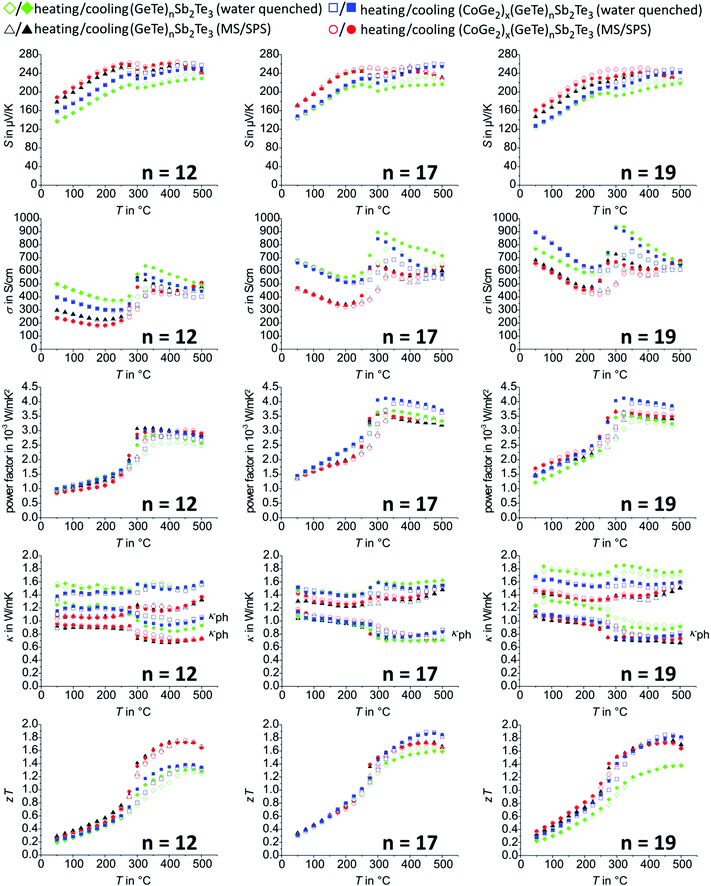 | ||
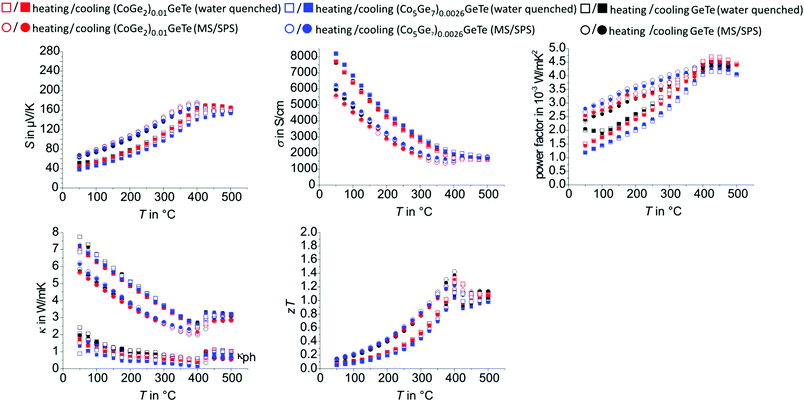
| Fig. 4 Thermoelectric properties of (GeTe)12Sb2Te3vs. (CoGe2)0.15(GeTe)12Sb2Te15 (left column), (GeTe)17Sb2Te3vs. (CoGe2)0.2(GeTe)17Sb2Te3 (middle column), and (GeTe)19Sb2Te3vs. (CoGe2)0.22(GeTe)19Sb2Te3 (right column): Seebeck coefficients (first row), electrical conductivities (second row), power factor (third row), thermal conductivities and corresponding phononic part (L = 1.5 + e(−|S|/116) where L is in 10−8 W Ω K−2 and S in μV K−1, fourth row), and zT values (fifth row). All data points of the MS/SPS synthesis route were merged from 3 cycles between 50 °C and 500 °C, not taking into account the first heating curve which is affected by relaxation of metastable states; the individual cycles of the MS/SPS samples with the best average zT values (GeTe)19Sb2Te3 and (CoGe2)0.22(GeTe)19Sb2Te3 are presented in Fig, S13 und S14, ESI;† note that red dots and black triangles often overlap (especially at n = 17), as they exhibit similar values. | ||
Thermoelectric properties
After a first heating step to 500 °C, which eliminates metastable states, especially concerning the vacancy distribution,75–77 all transport properties of heterostructured GST and GeTe samples with cobalt germanide precipitates remain stable over several cycles and are well reproducible with samples from further syntheses, whereas in some corresponding pristine compounds a minimal decrease in zT value is detectable over time (see below). The temperature evolution of Seebeck coefficients and electrical conductivities exhibits hystereses associated with phase transitions (Fig. 4 and 5, also cf. Section temperature-dependent phase transitions). In water-quenched GST samples, the cobalt germanide precipitates significantly influence the zT values. This is due to increased power factors and typically, most pronounced for water-quenched (CoGe2)0.22(GeTe)19Sb2Te22, due to lower thermal conductivity of pristine GST material (Fig. 4). With respect to water-quenched GeTe samples, however, the beneficial effect of the precipitates is negligible. This could be due to the fact that the average charge carrier concentrations of GeTe samples are higher than optimal and thus a heterostructuring approach is not very promising (see below). In addition, small amounts of Ge precipitates detected in GeTe might also reduce the influence of cobalt germanide precipitates. The precipitates have little influence on phonon scattering in water-quenched samples, most likely because their dimensions (Fig. 2 and Fig. S15 for water-quenched GeTe materials, ESI†) are not in the nanometer range. However, they influence the microstructure and lead to smaller grain and sub-grain sizes as exemplarily shown for (GeTe)19Sb2Te3 and the corresponding heterostructure with cobalt germanides in Fig. 1; this seems beneficial for lower thermal conductivity. In contrast, the influence of cobalt germanide precipitates on the thermoelectric properties of MS/SPS samples is almost negligible; single-phase samples have almost the same zT values as the corresponding heterostructured ones.The thermal and electrical conductivities of MS/SPS samples are lower than those of water-quenched ones as the (sub-)grain sizes of the GST and GeTe matrix is drastically reduced because of the rapid solidification conditions of the MS process, which might also induce strain. As the small (sub-)grain sizes of MS samples outweighs the effect of the precipitates on the GST matrix material, their influence on the thermoelectric properties of GST is much less pronounced for MS/SPS samples than for water-quenched ones. Thus, the precipitates influence the thermoelectric properties in a rather indirect way. Their mere presence does not significantly contribute to phonon scattering and thus does not significantly reduce κph even though their size is drastically reduced in MS/SPS samples. However, they influence the microstructure of the GST matrix, which explains their pronounced effect in water-quenched samples, whereas their effect cannot further enhance the beneficial effect of MS in terms of reducing the grain and/or subgrain size in the GST matrix. The cobalt germanides improve the long-term stability of the materials by stabilizing the microstructure by means of Zener pinning. Thus, the precipitates are also very beneficial in MS/SPS samples even if they do not enhance zT. In (CoGe2)0.22(GeTe)19Sb2Te3 (MS/SPS), zT values remain stable in several consecutive cycles up to 500 °C (Fig. S16, ESI†), whereas in pristine (GeTe)19Sb2Te3 (MS/SPS), κ increases and therefore zT gradually decreases upon thermal cycling (Fig. S17, ESI†). This can be explained by growing grains in pristine GST where the grain size is not stabilized by Zener pinning. Upon thermal cycling, increasing electrical and thermal conductivities and decreasing Seebeck coefficients result in slowly decreasing zT values.
However, the preparation of GeTe and its heterostructured variants by MS/SPS seems generally favorable in terms of zT value compared to corresponding water-quenched samples (Fig. 5). CoGe2 and Co5Ge7 precipitates (and a very small fraction of Ge precipitates in GeTe samples) were detected as mentioned in Section Phase composition and crystal structure. Formally adding 1 wt% of Co5Ge7 to GeTe (nominal composition) turns out not to significantly influence the thermoelectric properties compared to (CoGe2)0.01GeTe or GeTe. It turned out that heterostructuring GeTe with cobalt-containing precipitates is not very promising as discussed below.
The evaluation of charge carrier concentration and mobility by temperature-dependent Hall measurements gives further insights into optimal doping levels and scattering mechanisms. The application of an effective mass model (see formulae on the last page of the ESI†) reveals the theoretically possible maximum zT value for an optimal charge carrier concentration and thus the potential for further improvement via doping. Altogether, the approach of heterostructuring GeTe with cobalt germanides is less promising compared to GST hetero-structures, as the average carrier concentration of (CoGe2)0.01GeTe is much higher than optimal. Therefore, doping GeTe would have more impact than heterostructuring. In this respect, GST can be viewed as an optimally doped variant of GeTe. The Hall carrier mobility (Fig. 6) decreases with temperature, following a relationship between T−3/2 or T−1, which would correspond to phonon scattering in non-degenerate and degenerate semiconductors, respectively. As the curves exhibit small changes in slope, the exact exponent cannot be determined reliably and one may assume an intermediate type of behavior, which is quite typical in complex alloys. Both relationships are compatible with the fact that scattering of phonons, probably both acoustic and optical, limits the charge carrier mobility.78,79 Other forms of scattering, e.g. impurity scattering, would have a different temperature dependence. Therefore, the single parabolic band (SPB) model with the acoustic phonon scattering approximation was applied in the temperature range with an approximately linear increase of the Seebeck coefficient and the electrical resistivity, i.e. up to ca. 250 °C. With increasing Sb content, the Hall carrier mobility decreases. The effective charge carrier mass m* according to effective mass modelling of all three compositions changes only very little in the temperature range from 50 °C to 250 °C and no significant changes in charge carrier concentration and mobility were detected (cf.Fig. 6). This confirms that the precipitates do not lead to significant changes of the matrix by mutual doping. (CoGe2)0.15(GeTe)12Sb2Te3 and (CoGe2)0.22(GeTe)19Sb2Te3 exhibit almost optimal charge carrier concentrations. Therefore, (CoGe2)0.22(GeTe)19Sb2Te3 has the better dopant-independent intrinsic properties compared to (CoGe2)0.15(GeTe)12Sb2Te3 and thus the better average zT value in the temperature range from 50 °C to 250 °C. The best performing water-quenched samples with the composition (CoGe2)0.20(GeTe)17Sb2Te3 has the same average zT value of 1.17 as (CoGe2)0.22(GeTe)19Sb2Te3 (MS/SPS) – but is accessible in a more straightforward way.
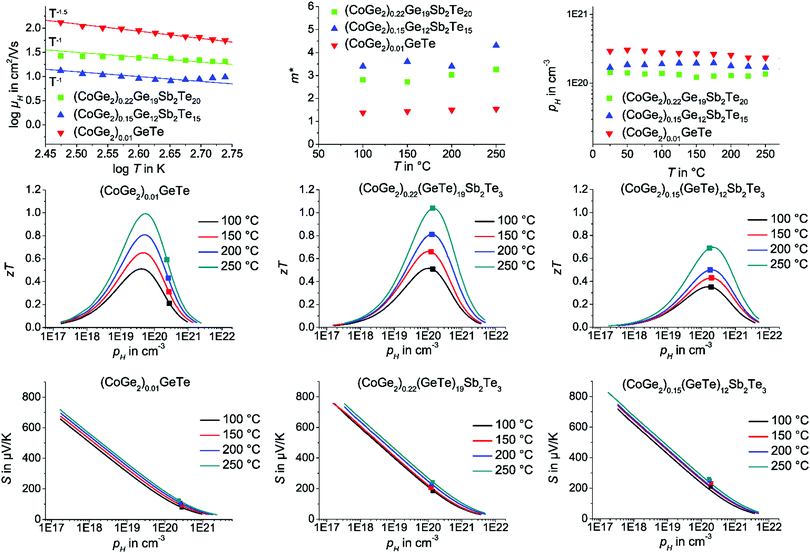 | ||
| Fig. 6 Slope of the Hall carrier mobility (top left), effective mass (top middle) and the Hall carrier concentration (top right) of MS/SPS samples of (CoGe2)0.01GeTe (red triangles), (CoGe2)0.15(GeTe)12Sb2Te3 (blue triangles) and (CoGe2)0.22(GeTe)19Sb2Te3 (green squares). For these samples, the calculated zT values as a function of pH at 100 °C (black line), 150 °C (red line), and 200 °C (blue line) and 250 °C (dark green) are shown (assuming SPB behavior, middle row), measured values depicted as squares; Seebeck coefficient as a function of log(pH) (Pisarenko plot, bottom row). Details of the calculations are described at the end of the ESI† and can be found in ref. 78 and 79. | ||
Conclusions
Comparing different synthesis routes, i.e. water-quenching and MS/SPS, the influence of cobalt germanide precipitates in GST materials on the thermoelectric properties turned out to be rather indirect and not a simple question of phonon scattering. In MS/SPS samples, the influence of heterostructuring GST materials with cobalt germanide precipitates on the thermoelectric properties is almost negligible, even though the cobalt germanide precipitates are significantly reduced in size from ∼1–2 μm (water-quenched) to <500 nm (MS/SPS). These nano-precipitates did not lead to a reduction of κph. Therefore, it is questionable to take the effect of nano-precipitates as effective phonon scattering centers for granted without any proof. Interestingly, some other studies also reported little influence of meltspinning on thermoelectric properties. For instance, precursors prepared by melt-spinning of Bi/Sb/Te alloys did not always enhance the thermoelectric performance of Bi2Te3-based materials due to the rapid grain growth during SPS; and PbTe with nanoscale MgTe precipitates obtaines by MS/SPS exhibits rather similar zT values compared to corresponding ingots.80,81SEM backscattered-electron orientation (“channeling”) contrast imaging with respect to the (sub-)grain size of the GST matrix material shows a significant difference in case of water-quenched samples. Introducing cobalt germanide precipitates into the GST matrix leads to an enhancement concerning thermoelectric performance of samples with microstructures that contain small grains and/or subgrains. Therefore, precipitates can be beneficial for the products of melt-casting syntheses by significantly reducing the grain, subgrain and domain sizes and would be an interesting approach for other materials to enhance their thermoelectric properties. This is especially true if the precipitates contain an element that is insoluble in the matrix, which prevents them from coarsening during annealing. Thus, Co-containing precipitates are an ideal way of heterostructuring GST materials. In MS/SPS samples, on the other hand, the grain size of the matrix material is mainly influenced by the rapid solidification conditions of MS, which leads to similar zT values of GST or GeTe and the corresponding heterostructured materials. MS/SPS samples exhibit a lower thermal conductivity, lower electrical conductivity combined with an increased Seebeck coefficient compared to water-quenched samples due to the reduced (sub-)grain size of the matrix material. In addition, heterostructuring may contribute to higher hardness (compare ref. 53) combined with an increased cyclability during thermoelectric measurements. We attribute the latter to the Zener pinning effect, which helps maintaining the (sub-)grain size of the matrix material and could be an interesting approach for future investigations in order to boost long-term stability of thermoelectric materials. As both synthesis routes lead to good thermoelectric materials, it has yet to be evaluated, which of the presented syntheses are better suited for upscaling, once these materials are considered for possible applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the European Union (European Social Fund, NFG ‘‘Effiziente Energienutzung: Neue Konzepte und Materialien’’) and the Deutsche Forschungsgemeinschaft (grant OE530/1-2) is gratefully acknowledged. S. Schwarzmüller thanks the Studienstiftung des deutschen Volkes for a fellowship. We thank, Samuel A. Miller for help with Hall measurements.Notes and references
- K. S. Siegert, F. R. L. Lange, E. R. Sittner, H. Volker, C. Schlockermann, T. Siegrist and M. Wuttig, Rep. Prog. Phys., 2015, 78, 013001 CrossRef CAS.
- M. Wuttig and N. Yamada, Nat. Mater., 2007, 6, 824–832 CrossRef CAS.
- T. Matsunaga, H. Morita, R. Kojima, N. Yamada, K. Kifune, Y. Kubota, Y. Tabata, J. J. Kim, M. Kobata, E. Ikenaga and K. Kobayashi, J. Appl. Phys., 2008, 103, 093511 CrossRef.
- C. Rios, M. Stegmaier, P. Hosseini, D. Wang, T. Scherer, C. D. Wright, H. Bhaskaran and W. H. P. Pernice, Nat. Photonics, 2015, 9, 725–732 CrossRef CAS.
- D. Lencer, M. Salinga and M. Wuttig, Adv. Mater., 2011, 23, 2030–2058 CrossRef CAS.
- J. Hegedüs and S. R. Elliott, Nat. Mater., 2008, 7, 399–405 CrossRef.
- M. N. Schneider, T. Rosenthal, C. Stiewe and O. Oeckler, Z. Kristallogr., 2010, 224, 463–470 Search PubMed.
- D. Lencer, M. Salinga and M. Wuttig, Adv. Mater., 2010, 225, 463–470 Search PubMed.
- D. Samson, T. Otterpohl, M. Kluge, U. Schmid and T. Becker, J. Electron. Mater., 2010, 39, 2092–2095 CrossRef CAS.
- D. Samson, M. Kluge, T. Fuss, U. Schmid and T. Becker, J. Electron. Mater., 2012, 41, 1134–1137 CrossRef CAS.
- D. Champier, Energy Convers. Manage., 2017, 140, 167–181 CrossRef.
- L. Kütt, J. Millar, A. Karttunen, M. Lehtonen and M. Karppinen, Renewable Sustainable Energy Rev., 2018, 98, 519–544 CrossRef.
- X. F. Zheng, C. X. Liu, Y. Y. Yan and Q. Wang, Renewable Sustainable Energy Rev., 2014, 32, 486–503 CrossRef CAS.
- L. C. Ding, A. Akbarzadeh and L. Tan, Renewable Sustainable Energy Rev., 2018, 81, 779–812 CrossRef.
- D. Beretta, N. Neophytou, J. M. Hodges, M. G. Kanatzidis, D. Narducci, M. Martin-Gonzalez, M. Beekman, B. Balke, G. Cerretti, W. Tremel, A. Zevalkink, A. I. Hofmann, C. Müller, B. Dörling, M. Coampoy-Quiles and M. Caironi, Mater. Sci. Eng., R. Rep., 2018 DOI:10.1016/j.mser.2018.09.001.
- E. Hazan, N. Madar, M. Parag, V. Casian, O. Ben-Yehuda and Y. Gelbstein, Adv. Electron. Mater., 2015, 11, 1500228 CrossRef.
- I. Cohen, M. Kaller, G. Komisarchik, D. Fuks and Y. Gelbstein, J. Mater. Chem. C, 2015, 3, 9559–9564 RSC.
- G. M. Guttmann, D. Dadon and Y. Gelbstein, J. Appl. Phys., 2015, 118, 065102 CrossRef.
- Y. Sadia, Z. Aminov, D. Mogilyansky and Y. Gelbstein, Intermetallics, 2016, 68, 71–77 CrossRef CAS.
- O. Appel and Y. Gelbstein, J. Electron. Mater., 2014, 43, 1976–1982 CrossRef CAS.
- P. Drude, Ann. Phys., 1900, 306, 566–613 CrossRef; P. Drude, Ann. Phys., 1900, 308, 369–402 CrossRef; P. Drude, Ann. Phys., 1902, 312, 687–692 CrossRef.
- H. J. Goldschmid, Introduction to Thermoelectricity, Springer, Heidelberg, 2009 Search PubMed.
- G. Wiedemann and R. Franz, Ann. Phys., 1853, 165, 497–531 CrossRef.
- H.-S. Kim, Z. M. Gibbs, Y. Tang, H. Wang and G. J. Snyder, APL Mater., 2015, 3, 041506 CrossRef.
- M. M. Koza, A. Leithe-Jasper, H. Rosner, W. Schnelle, H. Mutka, M. R. Johnson, M. Krisch, L. Capogna and Y. Grin, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 014306 CrossRef.
- G. A. Slack, in New Materials and Performance Limits for Thermoelectric Cooling, ed. D. M. Rowe, CRC Press, Boca Raton, 1995, ch. 34, pp. 407–440 Search PubMed.
- Y. Zhang and G. D. Stucky, Chem. Mater., 2014, 26, 837–848 CrossRef CAS.
- J. B. Williams and D. T. Morelli, J. Electron. Mater., 2017, 46, 2652–2661 CrossRef CAS.
- J. B. Williams and D. T. Morelli, J. Mater. Chem. C, 2016, 4, 10011–10017 RSC.
- K. Biswas, J. He, I. D. Blum, C.-I. Wu, T. P. Hogan, D. N. Seidman, V. P. Dravid and M. G. Kanatzidis, Nature, 2012, 489, 414–418 CrossRef CAS.
- K. F. Hsu, S. Loo, F. Guo, W. Chen, J. S. Dyck, J. C. Uher, T. Hogan, E. K. Polychroniadis and M. G. Kanatzidis, Science, 2004, 35, 818–821 CrossRef.
- J. Androulakis, K. F. Hsu, R. Pcionek, H. Kong, C. Uher, J. J. D'Angelo, A. Downey, T. Hogan and M. G. Kanatzidis, Adv. Mater., 2006, 18, 1170–1173 CrossRef CAS.
- Y. Pei, A. D. LaLonde, N. A. Heinz, X. Shi, S. Iwanaga, H. Wang, L. Chen and G. J. Snyder, Adv. Mater., 2011, 23, 5674–5678 CrossRef CAS.
- R. Hanus, M. T. Agne, A. J. E. Rettie, Z. Chen, G. Tan, D. Y. Chung, M. G. Kanatzidis, Y. Pei, P. W. Voorhees and G. J. Synder, Adv. Mater., 2019, 1900108 CrossRef.
- T. Rosenthal, P. Urban, K. Nimmrich, L. Schenk, J. de Boor, C. Stiewe and O. Oeckler, Chem. Mater., 2014, 26, 2567–2578 CrossRef CAS.
- R. Sankar, D. P. Wong, C.-S. Chi, W.-L. Chien, J. S. Hwang, F.-C. Chou, L. C. Chen and K. H. Chen, CrystEngComm, 2015, 17, 3440–3445 RSC.
- F. Yan, T. J. Zhu, X. B. Zhao and S. R. Dong, Appl. Phys. A: Mater. Sci. Process., 2007, 88, 425–428 CrossRef CAS.
- T. Rosenthal, M. N. Schneider, C. Stiewe, M. Döblinger and O. Oeckler, Chem. Mater., 2011, 23, 4349–4356 CrossRef CAS.
- S. Perumal, S. Roychowdhury and K. Biswas, J. Mater. Chem. C, 2016, 4, 7520–7536 RSC.
- H. S. Lee, B.-S. Kim, C.-W. Cho, M.-W. Oh, B.-K. Min, S.-D. Park and H.-W. Lee, Acta Mater., 2015, 91, 83–90 CrossRef CAS.
- S. Welzmiller, F. Heinke, P. Huth, G. Bothmann, E.-W. Scheidt, G. Wagner, W. Scherer, A. Pöppl and O. Oeckler, J. Alloys Compd., 2015, 652, 74–82 CrossRef CAS.
- T. Rosenthal, L. Neudert, P. Ganter, J. de Boor, C. Stiewe and O. Oeckler, J. Solid State Chem., 2014, 215, 231–240 CrossRef CAS.
- S. Welzmiller, F. Fahrnbauer, F. Hennersdorf, S. Dittmann, M. Liebau, C. Fraunhofer, W. G. Zeier, G. J. Snyder and O. Oeckler, Adv. Electron. Mater., 2015, 1, 1500266 CrossRef.
- T. Schröder, S. Schwarzmüller, C. Stiewe, J. de Boor, M. Hoelzel and O. Oeckler, Inorg. Chem., 2013, 52, 11288–11294 CrossRef.
- S. Schwarzmüller, M. Jakob, M. Nentwig, T. Schröder, A. Kuhn, A. Düvel, P. Heitjans and O. Oeckler, Chem. Mater., 2018, 30, 7970–7978 CrossRef.
- F. D. Rosi, J. P. Dismukes and E. F. Hockings, Electr. Eng., 1960, 79, 450–459 Search PubMed.
- S. Welzmiller, R. Schlegel, A. Poppl, G. Bothmann, E.-W. Scheidt, W. Scherer and O. Oeckler, Inorg. Chem., 2015, 641, 2350–2356 CAS.
- M. Nentwig, F. Fahrnbauer, M. Kasprick and O. Oeckler, J. Alloys Compd., 2017, 694, 1160–1164 CrossRef CAS.
- T. Rosenthal, S. Welzmiller and O. Oeckler, Solid State Sci., 2013, 25, 118–123 CrossRef CAS.
- M. Hong, Z.-G. Chen, L. Yang, Y.-C. Zou, M. S. Dargusch, H. Wang and J. Zou, Adv. Mater., 2018, 30, 1705942 CrossRef.
- Y. Gelbstein, J. Davidow, S. N. Girard, D. Y. Chung and M. G. Kanatzidis, Adv. Energy Mater., 2013, 3, 815–820 CrossRef CAS.
- D. Wu, L.-D. Zhao, S. Hao, Q. Jiang, F. Zheng, J. W. Doak, H. Wu, H. Chi, Y. Gelbstein, C. Uher, C. Wolverton, M. G. Kanatzidis and J. J. He, J. Am. Chem. Soc., 2014, 136, 11412–11419 CrossRef CAS.
- M. Samanta and K. Biswas, J. Am. Chem. Soc., 2017, 139, 9382–9391 CrossRef CAS.
- X. F. Zhang, H. Terasaki and Y. Komizo, Philos. Mag. Lett., 2011, 91, 491–497 CrossRef CAS.
- R. D. Doherty, D. A. Hughes, F. J. Humphreys, J. J. Jonas, D. Juul Jenson, M. E. Kassner, W. E. King, T. R. McNelley, H. J. McQueen and A. D. Rollett, Mater. Sci. Eng., A, 1997, 238, 219–274 CrossRef.
- W. C. Leslie, Physical metallurgy of steels, in Physical Metallurgy, ed. R. W. Cahn and P. Haasen, Elsevier Science B.V., Amsterdam, 1996 Search PubMed.
- K. S. Kakhramoanov, M. I. Zargarova and A. A. Mageramov, Inorg. Mater., 1981, 17, 26–29 Search PubMed.
- F. Fahrnbauer, D. Souchay, G. Wagner and O. Oeckler, J. Am. Chem. Soc., 2015, 137, 12633–12638 CrossRef CAS.
- F. Fahrnbauer, S. Maier, M. Grundei, N. Giesbrecht, M. Nentwig, T. Rosenthal, G. Wagner, G. J. Snyder and O. Oeckler, J. Mater. Chem. C, 2015, 40, 10525–10533 RSC.
- WINXPOW, v. 2.2.1, Stoe & Cie. GmbH, Darmstadt, Germany, 2007 Search PubMed.
- A. Coelho, TOPAS v. 5, Coelho Software, Brisbane, 2015 Search PubMed.
- P. W. Stephens, J. Appl. Crystallogr., 1999, 32, 281–289 CrossRef CAS.
- INCA, v. 4.02, Oxford Instruments Analytical Limited, Scotts Valley, USA, 1998–2007 Search PubMed.
- Genesis, v. 6.1, EDAX, Mahwah, USA, 2010 Search PubMed.
- analySIS, v. 2.1, Olympus Soft Imaging Solutions, Münster, Germany, 1996 Search PubMed.
- (a) P. A. Stadelmann, jEMS, v. 4.4631 U2016, CIME-EPFL, Lausanne, Switzerland, 2016 Search PubMed; (b) P. A. Stadelmann, Ultramicroscopy, 1987, 21, 131–145 Search PubMed.
- L. Dusza, High Temp. – High Pressures, 1995/1996, 27/28, 467–473 Search PubMed.
- K. A. Borup, E. S. Toberer, L. D. Zoltan, G. Nakatsukasa, M. Errico, J. P. Fleurial, B. B. Iversen and G. J. Snyder, Rev. Sci. Instrum., 2012, 83, 123902 CrossRef.
- M. N. Schneider, P. Urban, A. Leineweber, M. Döblinger and O. Oeckler, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 184102 CrossRef.
- M. N. Schneider, M. Seibald, P. Lagally and O. Oeckler, J. Appl. Crystallogr., 2010, 43, 1012–1020 CrossRef CAS.
- E. M. Levin, M. F. Besser and R. Hanus, J. Appl. Phys., 2013, 114, 083713 CrossRef.
- K. Schubert, T. R. Anantharaman, H. O. K. Ata, H. G. Meissner, M. Pötzschke, W. Rossteutscher and E. Stolz, Naturwissenschaften, 1960, 47, 512 CrossRef CAS.
- K. Cenzual, L. M. Gelato, M. Penzo and E. Parthe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 433–439 CrossRef.
- G. Gottstein and L. S. Shvindlerman, in Grain Boundary Migration in Metals: Thermodynamic, Kinetics, Applications, ed. B. Ralph, CRC Press, Boca Raton, 2nd edn, 2009 Search PubMed.
- P. Urban, M. N. Schneider and O. Oeckler, J. Solid State Chem., 2015, 227, 223–231 CrossRef CAS.
- M. N. Schneider, X. Biquard, C. Stiewe, T. Schröder, P. Urban and O. Oeckler, Chem. Commun., 2012, 48, 2192–2194 RSC.
- P. Urban, M. N. Schneider, M. Seemann, J. P. Wright and O. Oeckler, Z. Kristallogr., 2015, 230, 369–384 CAS.
- A. F. May and G. J. Snyder, in Thermoelectric and its Energy Harvesting, ed. D. M. Rowe, CRC Press, Boca Raton, 2012, ch. 11, pp. 1–18 Search PubMed.
- S. D. Kang and G. J. Snyder, arXiv:1710.06896v2.
- W. H. Shin, J. S. Yoon, M. Jeong, J. M. Song, S. Kim, J. W. Roh, S. Lee, W. S. Seo, S. W. Kim and K. H. Lee, Crystals, 2017, 7, 180 CrossRef.
- P. Eaksuwanchai, S. Tanusilp, P. Jood, M. Ohta and K. Kurosaki, ACS Appl. Energy Mater., 2018, 1, 6586 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of syntheses and sintering, density and Dulong-Petit Cp values, powder X-ray diffraction patterns, scanning and transmission electron microscopy (including EDX and SAED), and thermoelectric measurements. See DOI: 10.1039/c9tc03410b |
| This journal is © The Royal Society of Chemistry 2019 |