Anisotropic liquid metal–elastomer composites†
Abstract
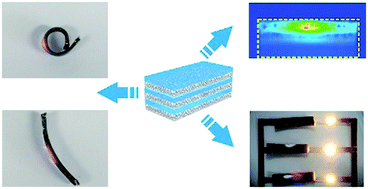
Anisotropic liquid metal (LM)–elastomer composites were fabricated by employing a sedimentation technique. The composite exhibits a remarkable electric anisotropy, heat transport anisotropy and mechanical anisotropy while being frozen. This composite can also be a shape morphing material to develop reconfigurable electronic circuits. This study opens up a powerful strategy for the design of LM–elastomer composites in responsive actuation, thermal regulation, and reconfigurable electronics.


 Please wait while we load your content...
Please wait while we load your content...