Probing the coupling of butterfly wing photonic crystals to plasmon resonances with surface-enhanced Raman spectroscopy†
Abstract
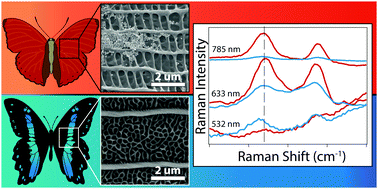
Both plasmonic and photonic materials manipulate light by structurally-defined optical properties. While photonic architectures can promote the scattering and reflection of certain wavelengths of light, plasmonic materials have plasmon resonances that confine light at the nanoscale. Coupling photonic materials to plasmon resonances could enhance the plasmonic response via wavelength-dependent interference and confinement properties. Here we explore the use of wavelength-dependent, naturally-abundant photonic crystal structures found in butterfly wings as substrates for plasmonic nanoparticle deposition, and probe the plasmonic–photonic interactions using surface-enhanced Raman spectroscopy. To better understand the wavelength dependence of field enhancement and localization of these systems, we examined the SERS responses of plasmonic nanoparticles deposited on four different butterfly wing colors with three excitation wavelengths. We find that excitation at wavelengths most closely matching the butterfly wing color produces the most intense SERS signal, with signal magnitude increases up to an order of magnitude, beyond a mere additive effect. These naturally abundant photonic structures show potential to create cheaper, wavelength-selective SERS substrates, and they provide a quantitative insight on plasmon–photonic crystal coupling.
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...
