Ultralow thermal conductivity of Tl4Ag18Te11†
Yixuan
Shi
a,
Nour
Mashmoushi
a,
Wojciech
Wegner
 ab,
Parisa
Jafarzadeh
a,
Zahra
Sepahi
a,
Abdeljalil
Assoud
a and
Holger
Kleinke
ab,
Parisa
Jafarzadeh
a,
Zahra
Sepahi
a,
Abdeljalil
Assoud
a and
Holger
Kleinke
 *a
*a
aDepartment of Chemistry, University of Waterloo, 200 University Ave W, Waterloo, ON N2L 3G1, Canada. E-mail: kleinke@uwaterloo.ca
bCollege of Inter-Faculty Individual Studies in Mathematics and Natural Sciences, University of Warsaw, Banacha 2C, 02-097 Warsaw, Poland
First published on 4th June 2019
Abstract
Tl4Ag18Te11 was reported to crystallize in a cubic structure with disordered thallium atoms filling cuboctahedral voids. Our electronic structure calculations of Tl4Ag18Te11 revealed a zero-band gap, based on an I4mm space group model with ordered Tl atom sites. Utilizing differential scanning calorimetry, we demonstrated that the material melted incongruently at 723 K. Our studies of the thermoelectric properties of hot-pressed Tl4Ag18Te11 and its nonstoichiometric homologs, revealed an unusual n-type extrinsic semiconducting behavior, combined with an ultralow thermal conductivity. The thermal conductivity of a sample of the nominal composition “Tl4.05Ag18Te11” reached 0.19 W m−1 K−1 at 500 K, with this value being one of the lowest achieved by crystalline materials to date. This can be attributed to the highly disordered Tl atoms and the large unit cell with its highly complex structure, in accord with the low Debye temperature of 98 K.
Introduction
Mankind continues to utilize fossil energy, which results in increased greenhouse gas emissions into the atmosphere. This consequently leads to further depletion of Earth's ozone layer and increases the rate of climate change. Thermoelectric materials offer a prospective solution by harvesting heat from different systems and converting it into useful energy without creating greenhouse gases.Currently, thermoelectric devices are primarily applied to produce electricity for satellites, aerospace machinery, communication systems, and powering daily life devices, such as wearable watches, fridges, and supplement energy to vehicles with internal combustion engines for fuel-efficient alternatives.1–3 Thermoelectric materials are evaluated by their figure-of-merit zT, based on the equation: zT = S2σT/κ, where S the Seebeck coefficient, σ the electrical conductivity, κ the thermal conductivity, and T the absolute temperature.4–7
Thallium chalcogenides have sparked continued interest in the thermoelectrics field because of their often low thermal conductivity,8,9 which is an important trait for advanced thermoelectric materials. Tl2Ag12Te7 was reported to achieve an ultralow thermal conductivity of 0.25 W m−1 K−1 and high zT of 1.1 at 525 K.10 TlAgTe possesses a low thermal conductivity of 0.36 W m−1 K−1 and zT of 0.6 at 550 K after substituting 40% of Ag with Cu.9 Tl8.1Pb1.9Te6 was stated to have a thermal conductivity of 0.45 W m−1 K−1 and a zTmax of 1.5 at 700 K.11 TlAg9Te5 demonstrated a thermal conductivity of 0.23 W m−1 K−1 and a high zT of 1.23 at 700 K.12 However, all of the above are p-type materials. There are only two n-type thallium tellurides reported thus far, namely TlBiTe2 and Tl2HgGeTe4, with only the thermoelectric properties of TlBiTe2 determined at high temperatures.13,14 Examples of state-of-the-art n-type thermoelectric materials include bismuth telluride,15,16 lead telluride,17,18 filled skutterudites19,20 and Bi-doped magnesium silicide stannides,21,22 all with zT values above unity. This paper introduces the hitherto unknown properties of the n-type Tl4Ag18Te11, which exhibits an ultralow thermal conductivity.
Experimental
The compounds were obtained via solid-state synthesis: stoichiometric amounts of the respective elements (Tl, granules, 6 mm, 99.99%, Alfa Aesar; Ag ingots, 100 mesh, 99.5%, Alfa Aesar; Te, broken ingots, 99.99%) were weighed and placed into a silica tube in an argon filled glove box. The tubes containing the elements were then transferred to a vacuum line and evacuated to 2.4 × 10−3 mbar. The tubes were sealed using a hydrogen/oxygen torch and then heated up to 923 K for several hours in a resistance furnace, and then water-quenched to room temperature. The yielded ingots were ground into fine powder and annealed at 573 K for 72 hours. The powder was then hot-pressed under a 5% hydrogen +95% argon atmosphere using the Oxy-Gon FR-210-30T-ASA-160-EVC hot press furnace system in a hardened graphite die with an inner diameter of 12.7 mm. The density of pellets was determined by the Archimedes method: the densities for all the presented samples were higher than 95% of the theoretical density (8.43 g cm−3).The electronic structure of Tl4Ag18Te11 was computed utilizing the WIEN2k package applying the full-potential linearized augmented plane wave (FP-LAPW) method with the density functional theory (DFT).23,24 The generalized gradient approximation (GGA) from Perdew, Burke and Ernzerhof (PBE) was utilized for exchange and correlation energies.25 Afterwards, the modified Becke–Johnson (mBJ) potential was applied to obtain a more reliable band gap. For the self-consistent energy calculations, 99 independent k points were selected on a grid of 10 × 10 × 10 points spread out evenly throughout the Brillouin zone. The energy convergence was set to be 10−4 Ry for the self-consistency.
The phase identification was performed utilizing our INEL X-ray powder diffractometer with a position-sensitive detector and Cu Kα1 radiation. The acceleration voltage and current were 30 kV and 30 mA, respectively. The diffraction patterns were collected at room temperature in the 2θ range from 5° to 120°.
The homogeneity of the samples was analysed by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDX) using a FEI Quanta Feg 250 ESEM with an acceleration voltage of 25 kV (WATLab, University of Waterloo). The experimental elemental ratios were determined by averaging four analyzed spots, and the standard deviations of the elemental ratios were determined by the population standard deviation method. No impurity elements were detected, and the sample appeared to be homogenous with Tl amounts generally slightly higher and Ag amounts slightly lower than expected based on the theoretical values. SEM images were also obtained on a hot-pressed pellet, showing a homogenous distribution of all elements (Fig. S1 in the ESI†).
Both differential scanning calorimetry (DSC) and thermogravimetry (TG) were performed with the Netzsch STA 409 PC Luxx instrument on an 80 mg powder sample of the nominal stoichiometry of Tl4Ag18Te11. The measurement was conducted under a constant argon flow with a heating rate of 20 K min−1 up to 773 K utilizing sapphire as the reference.
The thermal diffusivity, D, was measured on hot-pressed pellets with heights approximating 2 mm using the TA-Instruments LASERFLASH DLF-1 system under an ultrapure argon atmosphere. The thermal conductivity was determined via the equation κ = ρCpD, where ρ is the density of the pellet, and Cp is the specific heat, estimated through the Dulong–Petit law to be 0.198 J g−1 K−1, which is appropriate for high temperature values. The experimental error margin for our thermal conductivity measurements was estimated as ±5%. Low temperature specific heat measurements were performed with a Quantum Design PPMS Dynacool at WATLab. The experimentally determined Cp reached a plateau at 0.192 J g−1 K−1 (Fig. S2, ESI†), supporting the validity of using the Dulong–Petit value.
The Seebeck coefficient and electrical conductivity were determined by using our ULVAC-RIKO ZEM-3 system under ultrapure helium, utilizing a cut rectangular bar from the hot-pressed pellet with approximate dimensions of 10 × 2 × 2 mm. The experimental error for the Seebeck coefficient, electrical conductivity, and figure-of-merit are estimated as ±3%, ±5%, and ±10%.
Results and discussion
In 1992, Moreau et al. determined the crystal structure of Tl4Ag18Te11.26 This crystal structure consists of AgTe4 tetrahedra interconnected in three dimensions with Tl cations occupying tunnels of this framework (Fig. 1). The space group of Tl4Ag18Te11 is F![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3m, a subgroup of the K4Ag18Te11 space group Fm
3m, a subgroup of the K4Ag18Te11 space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m,27 due to the disordered Tl atom positions occupying an additional Wyckoff site. The lattice parameter of Tl4Ag18Te11 was published to be a = 18.717(8) Å, and the unit cell volume V = 6557.1 Å3. The Tl atom positions in the cubic (Tl1 atoms) and antiprismatic (Tl2 atoms) voids are 100% occupied, but disordered within the cuboctahedral (Tl3 atoms) voids. In K4Ag18Te11, all the potassium atoms were determined to be located in the centers of the respective polyhedra. However, the large anisotropic displacement parameter of the K3 atom (Ueq = 0.067(6) Å2, compared to K1 with 0.016(2) Å2 and K2 with 0.013(1) Å2), may indicate a tendency towards disorder in that cage as well.
m,27 due to the disordered Tl atom positions occupying an additional Wyckoff site. The lattice parameter of Tl4Ag18Te11 was published to be a = 18.717(8) Å, and the unit cell volume V = 6557.1 Å3. The Tl atom positions in the cubic (Tl1 atoms) and antiprismatic (Tl2 atoms) voids are 100% occupied, but disordered within the cuboctahedral (Tl3 atoms) voids. In K4Ag18Te11, all the potassium atoms were determined to be located in the centers of the respective polyhedra. However, the large anisotropic displacement parameter of the K3 atom (Ueq = 0.067(6) Å2, compared to K1 with 0.016(2) Å2 and K2 with 0.013(1) Å2), may indicate a tendency towards disorder in that cage as well.
 | ||
| Fig. 1 Left: Crystal structure of Tl4Ag18Te11. Blue polyhedra: AgTe4 tetrahedra; grey circles: disordered Tl3 atoms; right: arrangements of Tl atoms in the chain along the c-axis. | ||
The shortest distance between the Tl2 atom and the disordered Tl3 atom is 3.57 Å as a consequence of its displacement from the center of its polyhedron. Typical homonuclear Tl–Tl distances in thallium chalcogenides, such as Tl5Te3,28 Tl9BiTe6,29 and Tl4ZrTe4,30 are in the range of 3.5 Å to 4.0 Å. While the cuboctahedron cannot accommodate two Tl atoms, two Ag atoms would fit, with an Ag–Ag distance of 3.0 Å, thereby allowing for a cation excess and thus extra electrons causing n-type conduction.
The Tl1–Te distances in the Tl1Te6 cube are between 3.47 Å and 3.58 Å, and the Tl2–Te distances in the Tl2Te6 antiprism between 3.62 Å and 3.69 Å. On the other hand, the Tl3 atoms occupy the void in a cuboctahedron, whose center is too far away (5.04 Å) from the surrounding twelve Te atoms for regular Tl3–Te bonds. Consequently, the Tl3 positions were refined to be disordered in that cage, resulting in six sites, each with a 16.7% occupancy. Each of these sites participates in four short Tl3–Te bonds of 3.32 Å, and larger contacts with the next four Te atoms at a distance of 4.42 Å. This situation is reminiscent of the guest atoms in the icosahedral cages of the filled skutterudites31–33 and in the tetrakaidecahedral holes of the clathrates.8,34,35
The distances between all the Te atoms are larger than 4 Å, so their oxidation states are assigned as Tl+, Ag+, and Te2−, resulting in a charge balanced formula of (Tl+)4(Ag+)18(Te2−)11. The Ag atoms in this compound form Ag12 clusters that are three-dimensionally interconnected by Ag2 dumbbells. d10–d10 Ag–Ag interactions of the order of 3 Å are thus present in this structure, which are common in silver chalcogenides, such as Tl2Ag12Te7,10 Tl2Ag12Se7,36 K4Ag18Te11, and TlNdAg2S3.37
The PXRD patterns of the synthesized samples are presented in Fig. 2. No significant amount of impurities or side products were detected in any sample. The atomic percentages (at%) obtained by EDX analysis are presented in Table 1. The amount of Te is very close to the nominal percent for all the samples. The at% of Tl are between 0.4–0.7% higher and 0.3–0.5% lower than the theoretical percentages in case of Ag. The two samples with the same nominal formula reveal similar elemental ratios, indicating the samples are reproducible. The standard deviations are 0.1–0.6%, demonstrating that homogeneous samples were obtained.
 | ||
| Fig. 2 PXRD pattern of calculated Tl4Ag18Te11, experimental Tl4Ag18Te11, “Tl4.05Ag18Te11”, “Tl4Ag18Te10.95”, and repeat samples of the latter two. | ||
The electronic structure was determined based on an I4mm space group model, where one of the six disordered Tl3 sites was treated as 100% occupied, with the other five removed, as shown in Fig. 3(a). The density of states (DOS) of Tl4Ag18Te11 is shown in Fig. 3(b). From −6.5 eV to −3 eV, the DOS is dominated by Ag-d orbitals, from −2 eV to the Fermi level (EF), it is mainly composed of Te-p orbitals and Ag-d orbitals and above EF to 3 eV, the states contain (empty) Ag-s and Ag-p states, with some contributions from covalent mixing with Te-p states. The Tl states have only negligible contributions, partially caused by the small ratio of Tl in this material and the Tl-s states appearing to be below the selected energy range. The band structure (shown in Fig. 3(c)) reveals a zero band gap, with the valence band slightly in contact with the conduction band without any overlapping, notably known as the Dirac-semimetallic band structure. A similar band structure was determined for K4Ag18Te11, with zero band gap. The flat bands just below the Fermi energy and the steep bands just above the Fermi energy predict that this material could possibly possess a high Seebeck coefficient and a high electrical conductivity.
 | ||
| Fig. 3 (a) I4mm structure model; (b) density of states (DOS); (c) band structure of the I4mm model of Tl4Ag18Te11. | ||
The thermal behavior of Tl4Ag18Te11 was investigated by DSC and TG measurements, and the data is plotted in Fig. 4. The curve of TG indicates a weight gain smaller than 1%. The DSC curve reveals that Tl4Ag18Te11 melts incongruently starting from 723 K. According to the Tl2Te–Ag2Te phase diagram published in 1990,38 the melting processes can be interpreted as (with L = liquid):
| At 723 K: Tl4Ag18Te11 → TlAg5.14Te3.14 + L |
| At 729 K: TlAg5.14Te3.14 → TlAg9Te5 + L′ |
| At 780 K: TlAg9Te5 → Ag2Te + L′′ |
| (At 1228 K: Ag2Te → L′′′) |
However, the last process occurs outside of the temperature range we measured. The small exothermic peak presented at 570 K might correspond to the peritectic point of TlTe.39 The PXRD pattern obtained after this DSC measurement revealed a mixture of phases caused by the fast cooling process going through peritectic points.
All the physical properties obtained during cooling agree with the data obtained during heating, proving the data are reproducible (Fig. S3, ESI†). The Seebeck coefficients, S, of all samples are negative (Fig. 5(a)), which indicates that they are n-type semiconductors. This may be a consequence of a small amount of nonstoichiometric defects, such as two Ag cations replacing one Tl atom in the cuboctahedron of Te atoms, leading to a small amount of excess electrons.
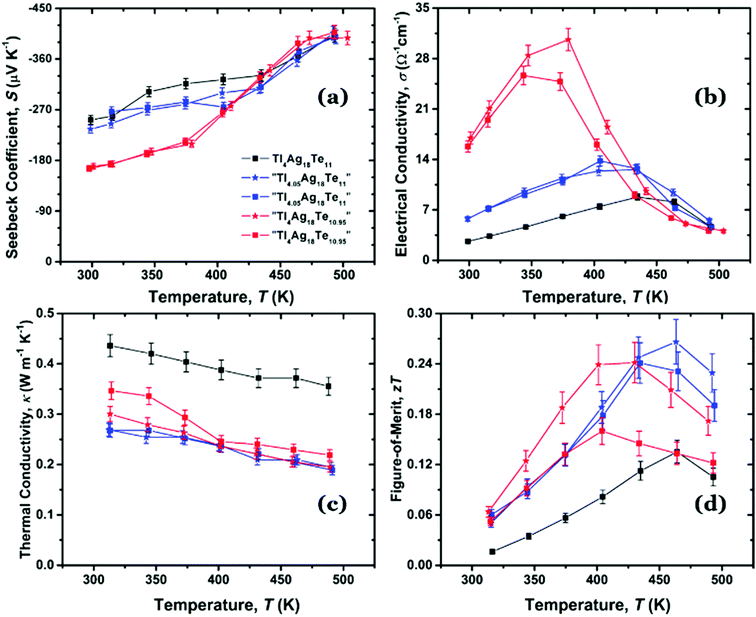 | ||
| Fig. 5 Physical properties of Tl4Ag18Te11. (a) Seebeck coefficient, (b) electrical conductivity, (c) thermal conductivity, and (d) figure-of-merit versus temperature. | ||
The absolute Seebeck values increase with a slower rate below 400 K, and then with a larger slope above 400 K. S of Tl4Ag18Te11 ranges from −250 μV K−1 at room temperature to −400 μV K−1 at 500 K. The absolute Seebeck values of “Tl4.05Ag18Te11” (with S = −240 μV K−1 at 300 K to S = −300 μV K−1 at 420 K) and “Tl4Ag18Te10.95” (S = −160 μV K−1 at 300 K to −270 μV K−1 at 420 K) are both lower compared to Tl4Ag18Te11 up to 420 K. Beyond that, all curves merge to equivalent values within experimental error. The maximum absolute Seebeck value of TlBiTe2 is much lower than the one of Tl4Ag18Te11 (with S = −75 μV K−1 at 760 K).13 The absolute Seebeck coefficient values of Tl4Ag18Te11 are also much higher than the values of Mg2Si0.325Sn0.645Bi0.03 (S = −115 μV K−1 at 300 K to −215 μV K−1 at 773 K),22 and the values of Bi2Te2.79Se0.21 (S = −115 μV K−1 at 300 K to −150 μV K−1 at 540 K).21
As displayed in Fig. 5(b), the electrical conductivity of stoichiometric Tl4Ag18Te11 increases as temperature increases to a maximum of σ = 9 Ω−1 cm−1 at 435 K, and then it starts to rapidly decrease. The four non-stoichiometric materials exhibit higher electrical conductivity at low temperature, indicative of more extrinsic charge carriers, in line with their lower absolute Seebeck values. For “Tl4.05Ag18Te11”, the electrical conductivity increases to a maximum of σ = 13 Ω−1 cm−1 at 435 K. For “Tl4Ag18Te10.95”, the electrical conductivity of the two different samples have some minor differences while generally exhibiting the same slope – the higher one reaches its electrical conductivity maximum value of 31 Ω−1 cm−1 at 380 K, which is almost 30 times lower than in TlBiTe2,13 40 times lower than in Bi2Te2.79Se0.21,21 and 70 times lower than in Mg2Si0.325Sn0.645Bi0.03.22 The differences between these two samples with the same nominal formula “Tl4Ag18Te10.95” may be caused by weighing errors leading to small differences in the actual composition. E.g., an error of 0.5 mg would correspond to 0.09% Tl or 0.05% Te. Above 425 K, the electrical conductivity curves of all materials merge together, because more intrinsic charge carriers are thermally activated, eliminating the differences in the initial carrier concentration.
The samples all possess very low thermal conductivity for the whole temperature range, and their thermal conductivity decreases as temperature increases due to increased phonon Umklapp scattering,40 as shown in Fig. 5(c). The thermal conductivity κ of Tl4Ag18Te11 ranges from 0.44 W m−1 K−1 at 320 K to 0.35 W m−1 K−1 at 500 K. The thermal conductivity of “Tl4.05Ag18Te11” is the lowest in this series, ranging from 0.27 W m−1 K−1 at 320 K to 0.19 W m−1 K−1 at 500 K. The two sets of thermal conductivity data obtained from the two different samples of “Tl4.05Ag18Te11” correspond with each other very well.
For comparison, these values are almost ten times lower than the thermal conductivity of TlBiTe2,13 17 times lower than in Mg2Si0.325Sn0.645Bi0.03 (κ = 3 W m−1 K−1 at 500 K),22 and nine times lower than in Bi2Te2.79Se0.21 (1.6 W m−1 K−1 at 500 K).21 The comparison of total thermal conductivity between “Tl4.05Ag18Te11” with five other state-of-the-art thermoelectric materials is presented in Fig. 6, demonstrating the thermal conductivity of “Tl4.05Ag18Te11” is extremely low. The range of thermal conductivity of bulk nanostructured Bi2Te3,41 Pb0.98Tl0.02Te,42 BiSbTe,43 Cu1.98Ag0.02Te,44 and Ag1−xPb18SbTe20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 is approximately 0.4–2.2 W m−1 K−1, and their maximum zT values all exceed unity.
45 is approximately 0.4–2.2 W m−1 K−1, and their maximum zT values all exceed unity.
The total thermal conductivity (κtot) contains both the lattice (κl) and electrical contributions (κe) to thermal conductivity: κtot = κl + κe, where κe can be estimated from the Wiedemann–Franz equation: κe= LσT.46 The Lorenz number L was calculated from the measured Seebeck coefficient values based on the single Kane band model.47 Due to the small electrical conductivity, the electrical thermal conductivity is only a minor contribution (its maximum value reaches 0.008 W m−1 K−1 at 430 K), and the lattice thermal conductivity is about 0.26 W m−1 K−1 at 320 K and 0.18 W m−1 K−1 at 500 K.
This ultralow thermal conductivity correlates nicely with the low Debye temperature of θD = 98 K obtained from of fit of the specific heat measurement. For comparison, PbTe has a Debye temperature of 177 K, and the binary thallium tellurides Tl2Te, Tl5Te3, TlTe, and Tl2Te3 all comparable values between 107 K and 124 K.48 The Debye temperature is directly proportional to the speed of sound:
Therein, ħ is the reduced Planck constant, kB the Boltzmann constant, n the number density of atoms {number of atoms/cell/(unit cell volume)}, and νS the arithmetic average of the speed of sound. νS in turn is proportional to the minimum thermal conductivity of both Clarke49 and Cahill,50 recently compared to the “diffuson thermal conductivity” by Snyder's group:51
| Cahill: κglass = 1.21n2/3kBνS |
| Clarke: κmin = 0.93n2/3kBνS |
| Snyder: κdiff = 0.76n2/3kBνS |
To derive the equation for κdiff, thermal transport was based on diffusons instead of on phonons as used for κglass. This results in lower values for all materials, and may well correspond to the lowest possible thermal conductivity. Using νS = 1385 m s−1 as calculated from θD = 98 K, we obtain κglass = 0.27 W m−1 K−1, κmin = 0.21 W m−1 K−1, and κdiff = 0.17 W m−1 K−1. These numbers show that “Tl4.05Ag18Te11” exhibits a thermal conductivity below the so-called glass limit according to Cahill, but not below the diffuson limit.
This extraordinary low thermal conductivity is likely caused by Tl disorders, heavy composing elements, and the large unit cell, which all contribute to reducing the mean free path length of the phonons travelling though the lattice. The disorder effect to lower thermal conductivity was thoroughly discussed in many previously published articles, such as for Tl2Ag12Te7+δ,10 Cu2Se,52 and Ba3Cu16−x(S,Te)11.53 The “cage rattling” mechanism, where Tl3 atoms exhibit concerted rattling in a cage of Te atoms, is known to be important as well. Best known examples of this phenomenon are the filled skutterudites54–56 and clathrates.8,34,35,57 This concerted rattling phenomenon was also observed in CsAg5Te3, which owns an ultralow lattice thermal conductivity of 0.14 W m−1 K−1 at 727 K, and a Debye temperature of 118 K.58 Furthermore, heavy elements tend to vibrate with a smaller amplitude because of the inertial resistance provided by the heavy nucleus, and thus cause a lower phonon transfer effect to the neighboring atoms. The principles of how larger unit cells and more complex crystal structures lower the lattice thermal conductivity were reviewed in 2010.59
The figure-of-merit curves of the samples are plotted in Fig. 5(d). The stoichiometric Tl4Ag18Te11 sample achieves a maximum zT value of 0.13 at 470 K; whereas the zT of “Tl4.05Ag18Te11” is twice as large, reaching a maximum of 0.26 at 465 K. The highest zT of “Tl4.05Ag18Te11” is higher than the maximum zT of TlBiTe2 which was achieved at 760 K with a value of 0.15.13 However, it is still low in comparison to Mg2Si0.325Sn0.645Bi0.03, whose maximum zT is 1.35 at 773 K,22 and Bi2Te2.79Se0.21 whose maximum zT is 0.9 at 430 K.21 For the two “Tl4Ag18Te10.95” samples, the one with higher electrical conductivity exhibits the higher zT of 0.24 between 400 K and 425 K. These relatively low zT values could likely be significantly improved if the carrier concentration could be optimized via doping with heteroelements.
Conclusions
Thallium chalcogenides are known to rarely perform as n-type semiconductors; however, we found that Tl4Ag18Te11 is an n-type material. The thermal behaviour of Tl4Ag18Te11 was investigated by DSC, illustrating its incongruent melting at 723 K. The samples prepared and investigated are homogenous, and the physical properties of the samples are reproducible. All the samples have negative Seebeck coefficient values throughout the temperature range of 300–500 K, indicating that the majority of charge carriers are electrons. “Tl4.05Ag18Te11” has the lowest thermal conductivity of 0.19 W m−1 K−1 at 500 K, which is extraordinary low compared to other thermoelectric materials. This is mainly a consequence of the disordered heavy Tl atoms filling the cuboctahedral cages and the complex crystal structure built by heavy elements.The highest dimensionless figure-of-merit, zT, is about 0.26 obtained by the sample with the nominal formula of “Tl4.05Ag18Te11” at 475 K, which is twice as high as the maximum zT of Tl4Ag18Te11. Doping with other elements in order to optimize the thermoelectric properties, which are at this moment negatively affected by the low electrical conductivity, will be performed in the near future. Overall, Tl4Ag18Te11 based materials will provide a promising n-type thermoelectric material, if their carrier concentrations can be enhanced.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Natural Sciences and Engineering Research Council of Canada in form of a Discovery Grant (RGPIN-2015-04584) is appreciated.References
- J. Yang and F. R. Stabler, Automotive Applications of Thermoelectric Materials, J. Electron. Mater., 2009, 38(7), 1245–1251 CrossRef CAS.
- L. E. Bell, Cooling, heating, generating power, and recovering waste heat with thermoelectric systems, Science, 2008, 321(5895), 1457–1461 CrossRef CAS PubMed.
- I. Petsagkourakis, K. Tybrandt, X. Crispin, I. Ohkubo, N. Satoh and T. Mori, Thermoelectric materials and applications for energy harvesting power generation, Sci. Technol. Adv. Mater., 2018, 19(1), 836–862 CrossRef PubMed.
- G. J. Snyder and E. S. Toberer, Complex thermoelectric materials, Nat. Mater., 2008, 7(2), 105–114 CrossRef CAS PubMed.
- Y. Shi, C. Sturm and H. Kleinke, Chalcogenides as thermoelectric materials, J. Solid State Chem., 2019, 270, 273–279 CrossRef CAS.
- J. He and T. M. Tritt, Advances in thermoelectric materials research: Looking back and moving forward, Science, 2017, 357(6358), eaak9997 CrossRef PubMed.
- T. Mori, Novel Principles and Nanostructuring Methods for Enhanced Thermoelectrics, Small, 2017, 13(45), 1702013 CrossRef PubMed.
- H. Kleinke, New bulk materials for thermoelectric power generation: clathrates and complex antimonides, Chem. Mater., 2010, 22(3), 604–611 CrossRef CAS.
- K. Kurosaki, K. Goto, H. Muta and S. Yamanaka, Enhancement of thermoelectric figure of merit of AgTlTe by tuning the carrier concentration, J. Appl. Phys., 2007, 102(2), 023707 CrossRef.
- Y. Shi, A. Assoud, S. Ponou, S. Lidin and H. Kleinke, A New Material with a Composite Crystal Structure Causing Ultralow Thermal Conductivity and Outstanding Thermoelectric Properties: Tl2Ag12Te7+δ, J. Am. Chem. Soc., 2018, 140(27), 8578–8585 CrossRef CAS PubMed.
- Q. Guo, A. Assoud and H. Kleinke, Improved bulk materials with thermoelectric figure-of-merit greater than 1: Tl10−xSnxTe6 and Tl10−xPbxTe6, Adv. Energy Mater., 2014, 4(14), 1400348 CrossRef.
- K. Kurosaki, A. Kosuga, H. Muta, M. Uno and S. Yamanaka, Ag9TlTe5: A high-performance thermoelectric bulk material with extremely low thermal conductivity, Appl. Phys. Lett., 2005, 87(6), 3–5 CrossRef.
- K. Kurosaki, A. Kosuga and S. Yamanaka, Thermoelectric properties of TlBiTe2, J. Alloys Compd., 2003, 351(1–2), 279–282 CrossRef CAS.
- M. A. McGuire, T. J. Scheidemantel, J. V. Badding and F. J. DiSalvo, Tl2AXTe4 (A = Cd, Hg, Mn; X = Ge, Sn): Crystal Structure, Electronic Structure, and Thermoelectric Properties, Chem. Mater., 2005, 17(24), 6186–6191 CrossRef CAS.
- X. Yan, B. Poudel, Y. Ma, W. S. Liu, G. Joshi and H. Wang, et al., Experimental studies on anisotropic thermoelectric properties and structures of n-type Bi2Te2.7Se0.3, Nano Lett., 2010, 10(9), 3373–3378 CrossRef CAS PubMed.
- L. P. Hu, X. H. Liu, H. H. Xie, J. J. Shen, T. J. Zhu and X. B. Zhao, Improving thermoelectric properties of n-type bismuth-telluride-based alloys by deformation-induced lattice defects and texture enhancement, Acta Mater., 2012, 60(11), 4431–74431 CrossRef CAS.
- L. Yang, Z. G. Chen, M. Hong, L. Wang, D. Kong and L. Huang, et al., n-Type Bi-doped PbTe Nanocubes with Enhanced Thermoelectric Performance, Nano Energy, 2017, 31, 105–112 CrossRef CAS.
- A. D. Lalonde, Y. Pei and G. J. Snyder, Reevaluation of PbTe1-xIx as high performance n-type thermoelectric material, Energy Environ. Sci., 2011, 4(6), 2090–2096 RSC.
- M. Matsubara and R. Asahi, Optimization of Filler Elements in CoSb3-Based Skutterudites for High-Performance n-Type Thermoelectric Materials, J. Electron. Mater., 2016, 45(3), 1669–1678 CrossRef CAS.
- G. Rogl, A. Grytsiv, K. Yubuta, S. Puchegger, E. Bauer and C. Raju, et al., In-doped multifilled n-type skutterudites with ZT = 1.8, Acta Mater., 2015, 95, 201–211 CrossRef CAS.
- L. Hu, H. Wu, T. Zhu, C. Fu, J. He and P. Ying, et al., Tuning Multiscale Microstructures to Enhance Thermoelectric Performance of n-Type Bismuth-Telluride-Based Solid Solutions, Adv. Energy Mater., 2015, 5(17), 1500411 CrossRef.
- L. R. Macario, X. Cheng, D. Ramirez, T. Mori and H. Kleinke, Thermoelectric Properties of Bi-Doped Magnesium Silicide Stannides, ACS Appl. Mater. Interfaces, 2018, 10, 40585–40591 CrossRef CAS PubMed.
- P. Blaha, K. Schwarz, G. K. H. Madsen, D. Kvasnicka and J. Luitz, WIEN2k, An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties, ed. K. Schwarz, Techn. Universität, Wien, Austria, 2001 Search PubMed.
- K. Schwarz, DFT calculations of solids with LAPW and WIEN2k, J. Solid State Chem., 2003, 176, 319–328 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77(18), 3865–3868 CrossRef CAS PubMed.
- J. Le Roy, J. M. Moreau, G. Brun and B. Liautard, A new phase in the Tl-Ag-Te system: crystal structure of Tl4Ag24−xTe15−y, J. Alloys Compd., 1992, 186(2), 249–254 CrossRef CAS.
- B. Davaasuren, E. Dashjav and A. Rothenberger, Synthesis and Characterization of the Ternary Telluroargentate K4[Ag18Te11], Z. Anorg. Allg. Chem., 2014, 640(14), 2939–2944 CrossRef CAS.
- I. Schewe, P. Böttcher and H. G. von Schnering, The crystal structure of Tl5Te3 and its relationship to the Cr5B3 type, Z. Kristallogr., 1989, 188, 287–298 CrossRef CAS.
- Q. Guo, M. Chan, B. A. Kuropatwa and H. Kleinke, Enhanced thermoelectric properties of variants of Tl9SbTe6 and Tl9BiTe6, Chem. Mater., 2013, 25(20), 4097–4104 CrossRef CAS.
- C. R. Sankar, S. Bangarigadu-Sanasy, A. Assoud and H. Kleinke, Syntheses, Crystal Structures and Thermoelectric Properties of two new Thallium Tellurides: Tl4ZrTe4 and Tl4HfTe4, J. Mater. Chem., 2010, 20, 7485–7490 RSC.
- J. Eilertsen, S. Rouvimov and M. A. Subramanian, Rattler-seeded InSb nanoinclusions from metastable indium-filled In0.1Co4Sb12 skutterudites for high-performance thermoelectrics, Acta Mater., 2012, 60(5), 2178–2185 CrossRef CAS.
- C. H. Lee, I. Hase, H. Sugawara, H. Yoshizawa and H. Sato, Low-lying optical phonon modes in the filled skutterudite CeRu4Sb12, J. Phys. Soc. Jpn., 2006, 75(12), 123602 CrossRef.
- G. A. Slack, Design concepts for improved thermoelectric materials, Mater. Res. Soc. Symp. Proc., 1997, 478, 47–54 CrossRef CAS.
- M. Christensen, S. Johnsen and B. B. Iversen, Thermoelectric clathrates of type I, Dalton Trans., 2010, 39(4), 978–992 RSC.
- T. Takabatake, K. Suekuni, T. Nakayama and E. Kaneshita, Phonon-glass electron-crystal thermoelectric clathrates: Experiments and theory, Rev. Mod. Phys., 2014, 86, 669–716 CrossRef CAS.
- Y. Shi, A. Assoud, C. R. Sankar and H. Kleinke, Tl2Ag12Se7: a new pnp conduction switching material with extraordinarily low thermal conductivity, Chem. Mater., 2017, 29(21), 9565–9571 CrossRef CAS.
- A. Assoud, Y. Shi, Q. Guo and H. Kleinke, Crystal and electronic structure of the new quaternary sulfides TlLnAg2S3 (Ln = Nd, Sm and Gd), J. Solid State Chem., 2017, 256, 6–9 CrossRef CAS.
- G. Brun, M. Boubali, J. C. Tedenac, R. M. Ayral and B. Legendre, Redetermination of the phase equilibria in the system Tl2Te-Ag2Te, Thermochim. Acta, 1990, 165, 93–101 CrossRef CAS.
- A. A. Toure, G. Kra, R. Eholie, J. Olivier-Fourcade and J. C. Jumas, Mise au point sur le système binaire TlTe et affinement des structures de TlTe et Tl5Te3, J. Solid State Chem., 1990, 87, 229–236 CrossRef CAS.
- P. G. Klemens, Thermal resistance due to point defects at high temperatures, Phys. Rev., 1960, 119, 507 CrossRef CAS.
- M. Saleemi, M. S. Toprak, S. Li and M. Johnsson, Muhammed M. Synthesis, processing, and thermoelectric properties of bulk nanostructured bismuth telluride (Bi2Te3), J. Mater. Chem., 2012, 22(2), 725–730 RSC.
- J. P. Heremans, V. Jovovic, E. S. Toberer, A. Saramat, K. Kurosaki and A. Charoenphakdee, et al., Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States, Science, 2008, 321, 554–557 CrossRef CAS PubMed.
- B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich and B. Yu, et al., High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys, Science, 2008, 320, 634–638 CrossRef CAS PubMed.
- S. Ballikaya, H. Chi, J. R. Salvador and C. Uher, Thermoelectric properties of Ag-doped Cu2Se and Cu2Te, J. Mater. Chem. A, 2013, 1, 12478–12484 RSC.
- K. F. Hsu, S. Loo, F. Guo, W. Chen, J. S. Dyck and C. Uher, et al., Cubic AgPbmSbTe2+m: Bulk Thermoelectric Materials with High Figure of Merit, Science, 2004, 303(5659), 818–821 CrossRef CAS PubMed.
- G. V. Chester and A. Thellung, The Law of Wiedemann and Franz, Proc. Phys. Soc., 1961, 77, 1005 CrossRef CAS.
- Y. Pei, A. D. Lalonde, H. Wang and G. J. Snyder, Low effective mass leading to high thermoelectric performance, Energy Environ. Sci., 2012, 5, 7963–7969 RSC.
- H. Matsumoto, K. Kurosaki, H. Muta and S. Yamanaka, Thermoelectric Properties of the Thallium–Tellurium Binary Compounds, Mater. Trans., 2009, 50, 1582–1585 CrossRef CAS.
- D. R. Clarke, Materials selection guidelines for low thermal conductivity thermal barrier coatings, Surf. Coat. Technol., 2003, 163–164, 67–74 CrossRef CAS.
- D. G. Cahill, S. K. Watson and R. O. Pohl, Lower limit to the thermal conductivity of disordered crystals, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46(10), 6131–6140 CrossRef CAS.
- M. T. Agne, R. Hanus and G. J. Snyder, Minimum thermal conductivity in the context of diffuson-mediated thermal transport, Energy Environ. Sci., 2018, 11(3), 609–616 RSC.
- B. Yu, W. Liu, S. Chen, H. Wang, H. Wang and G. Chen, et al., Thermoelectric properties of copper selenide with ordered selenium layer and disordered copper layer, Nano Energy, 2012, 1(3), 472–478 CrossRef CAS.
- P. Jafarzadeh, M. Oudah, A. Assoud, N. Farahi, E. Müller and H. Kleinke, High thermoelectric performance of Ba3Cu16−x(S,Te)11, J. Mater. Chem. C, 2018, 6(47), 13043–13048 RSC.
- W. Y. Zhao, C. L. Dong, P. Wei, W. Guan, L. S. Liu and P. C. Zhai, et al., Synthesis and high temperature transport properties of barium and indium double-filled skutterudites BaxInyCo4Sb12-z, J. Appl. Phys., 2007, 102(11), 113708 CrossRef.
- C. Uher Skutterudites: prospective novel thermoelectrics. Semicond Semimet. 2001;69(Recent Trends in Thermoelectric Materials Research I):139–253.
- T. He, J. Chen, H. D. Rosenfeld and M. A. Subramanian, Thermoelectric Properties of Indium-Filled Skutterudites, Chem. Mater., 2006, 18, 759–762 CrossRef CAS.
- V. L. Kuznetsov, L. A. Kuznetsova, A. E. Kaliazin and D. M. Rowe, Preparation and thermoelectric properties of A8IIB16IIIB30IV clathrate compounds, J. Appl. Phys., 2000, 87(11), 7871–7875 CrossRef CAS.
- H. Lin, G. Tan, J. N. Shen, S. Hao, L. M. Wu and N. Calta, et al., Concerted Rattling in CsAg5Te3 Leading to Ultralow Thermal Conductivity and High Thermoelectric Performance, Angew. Chem., Int. Ed., 2016, 55(38), 11431–11436 CrossRef CAS PubMed.
- E. S. Toberer, A. F. May and G. J. Snyder, Zintl chemistry for designing high efficiency thermoelectric materials, Chem. Mater., 2010, 22(3), 624–634 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: One figure with SEM images, one figure showing the specific heat, three figures showing the stability of the materials. See DOI: 10.1039/c9tc02029b |
| This journal is © The Royal Society of Chemistry 2019 |







