Directed polymorphism and mechanofluorochromism of conjugated materials through weak non-covalent control†
Abstract
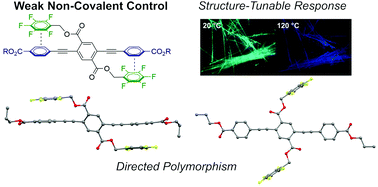
Understanding and manipulating crystal polymorphism can provide novel strategies for materials discovery in organic optoelectronics. In this paper, a series of seven ester-terminated three-ring phenylene ethynylenes (PEs) exhibit structure-dependent polymorphism wherein alkyl chain length modulates the propensity to form violet or green fluorescent solid phases, as well as tunable thermal and mechanofluorochromic (MFC) transitions. These compounds harness “soft” non-covalent control to achieve polymorphism: the electronic substituent effect of the ester groups weakens the fluoroarene–arene (ArF–ArH) interactions that typically direct crystal packing of this class of compounds, increasing competitiveness of other interactions. Small structural modifications tip this balance and shift the prevalence of violet- or green-emitting polymorphs. Compounds with short alkyl chain lengths show both violet and various green fluorescent polymorphs, while the violet fluorescent form dominates with alkyl lengths longer than butyl. Further, thermally induced green-to-violet fluorescent crystal-to-crystal transitions occur for single crystals of two derivatives. Finally, the PEs show reversible violet-to-green mechanofluorochromism (MFC), with temperature required for reversion of this MFC decreasing with alkyl chain length. We therefore present this design of directional but weak interactions as a strategy to access polymorphs and tunable stimuli-responsive behavior in solids.


 Please wait while we load your content...
Please wait while we load your content...