Photochemical synthesis of π-extended ullazine derivatives as new electron donors for efficient conjugated D–A polymers†
Abstract
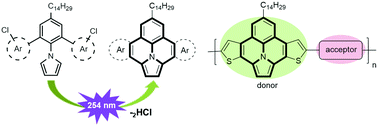
We report the synthesis of π-extended ullazine derivatives annulated with either electron-poor pyridine or electron-rich thiophene units through a metal-free, photochemical cyclodehydrochlorination (CDHC) reaction. The strongest electron-donor derivative, 7-tetradecylthieno[3′,2′:7,8]indolizino[6,5,4,3-ija]thieno[2,3-c]quinolone, was copolymerized with electron-deficient thienopyrroledione (TPD), isoindigo (IID), and diketopyrrolopyrrole (DPP) derivatives to provide three donor–acceptor conjugated polymers (D–A CPs). Their photophysical, electrochemical and photovoltaic (PV) properties were investigated. The polymers showed broad UV-vis-NIR absorption bands with λmax values of 612 nm, 698 nm, 788 nm in chloroform and exhibited optical bandgap (Eoptg) of 1.58 eV, 1.41 eV, 1.24 eV measured as films. Inverted bulk heterojunction polymer solar cells (BHJ-PSCs) were fabricated using these polymers as host and light-harvesting materials. The device based on P3:PC70BM blends shows the best power conversion efficiency (PCE) of 2.23% (Voc = 0.55 V, Jsc = 7.86 mA cm−2, FF = 52%). These promising results demonstrate that π-extended ullazine derivatives can be used as electron-rich building blocks for the construction of D–A CPs for efficient PSCs applications.


 Please wait while we load your content...
Please wait while we load your content...