Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation†
Abstract
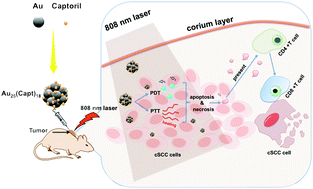
Phototherapy, particularly photothermal therapy (PTT) and photodynamic therapy (PDT), has become a promising therapeutic technique for the treatment of skin cancers because of its minial invasiveness, high efficacy, and low side effects. Nevertheless, single modality therapy, either PTT or PDT, has limited clinical effectiveness in treating skin cancers. Thus, combined applications of PTT and PDT have been frequently reported; however, PTT and PDT often require their respective photoagents and excitation light sources, resulting in challenges in clinical transformation. In this study, to address these issues, we report the use of biocompatible gold nanoclusters Au25(Capt)18 for the concurrent PTT and PDT treatment of cutaneous squamous cell carcinoma (cSCC) using an 808 nm near-infrared (NIR) laser. Utilizing their high light-thermal conversion efficiency, potent generation of singlet oxygen, and strong photothermal stability, Au25(Capt)18 nanoclusters potentiated a significant proliferation suppression of cSCC XL50 cells in vitro and the inhibition of cSCC tumors on SKH-1 mice in vivo. In particular, under 808 nm light irradiation, the tumor-cell-killing contributions of PTT and PDT were estimated to be 28.86% and 71.14%, respectively, by using an ROS scavenger to quench the PDT effect. Tumor-infiltrating CD4+ T and CD8+ T cells were observed after one course of concurrent PTT and PDT. Preliminary toxicity studies indicated low adverse effects of the Au25(Capt)18 nanoclusters. Through this study, we report the use of a simple nanostructure for simultaneous PTT and PDT applications to effectively kill cSCC and to induce anti-tumor immune responses. Our study could lead to the development of effective photoagents for current, synergistic applications of different phototherapies with targeted immunological responses for the treatment of cancers.


 Please wait while we load your content...
Please wait while we load your content...