Bifunctional MIL-53(Fe) with pyrophosphate-mediated peroxidase-like activity and oxidation-stimulated fluorescence switching for alkaline phosphatase detection†
Abstract
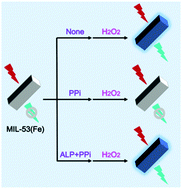
Alkaline phosphatase (ALP) is extensively used as a clinical biomarker because of its close relevance with a variety of diseases. Thus, exploring reliable and practical methods for ALP analysis is of great significance. In the present work, we explored MIL-53(Fe) as a bifunctional platform with pyrophosphate (PPi)-mediated peroxidase-like activity and oxidation-stimulated fluorescence switching for ALP sensing. The proposed MIL-53(Fe) could exhibit favorable peroxidase-mimicking activity to catalytically decompose H2O2 to hydroxyl radicals, which had strong oxidizing ability to oxidize the terephthalic acid bridging ligand, resulting in the oxidation-stimulated turn-on fluorescence of MIL-53(Fe) itself. Due to the strong coordination interaction between PPi and Fe3+, the former with a relatively large molecular structure was able to inhibit the catalytic activity of MIL-53(Fe) via capping active Fe3+ sites, leading to the suppression of its self-fluorescence response. When ALP was present, it could hydrolyze the PPi inhibitor and restore the dual functions of MIL-53(Fe) to provide fluorescence again. With the above principle, highly sensitive and selective determination of ALP with a linear scope of 2–80 U L−1 and a detection limit down to 0.7 U L−1 was achieved. The MIL-53(Fe) was also demonstrated to be very reliable in measuring the target in human serum, indicating its great promise as an integrated tool for ALP detection in clinical practice.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry B Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...