Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: copper and strontium
Lukas
Gritsch
 abe,
Muhammad
Maqbool
ab,
Viviana
Mouriño
cd,
Francesca E.
Ciraldo
a,
Mark
Cresswell
b,
Philip R.
Jackson
b,
Christopher
Lovell
b and
Aldo R.
Boccaccini
abe,
Muhammad
Maqbool
ab,
Viviana
Mouriño
cd,
Francesca E.
Ciraldo
a,
Mark
Cresswell
b,
Philip R.
Jackson
b,
Christopher
Lovell
b and
Aldo R.
Boccaccini
 *a
*a
aInstitute of Biomaterials, Friedrich-Alexander-University of Erlangen-Nuremberg, Cauerstraße 6, 91058 Erlangen, Germany. E-mail: aldo.boccaccini@ww.uni-erlangen.de
bLucideon Ltd, Queens Road, Penkhull, Stoke-on-Trent, Staffordshire ST4 7LQ, UK
cUniversidad de Buenos Aires, Pharmaceutical Technology Department, Buenos Aires, Argentina
dCONICET (National Research Council), Argentina
eLaboratoire de Physique de Clermont (LPC), Institut National de Physique Nucléaire et de Physique des Particules (CNRS/IN2P3), Université Clermont Auvergne, 4 avenue Blaise Pascal, 63178 Aubière, France. E-mail: lukas.gritsch@clermont.in2p3.fr
First published on 24th September 2019
Abstract
Therapeutic metal ions are a family of metal ions characterized by specific biological properties that could be exploited in bone tissue engineering, avoiding the use of expensive and potentially problematic growth factors and other sensitive biomolecules. In this work, we report the successful preparation and characterization of two material platforms containing therapeutic ions: a copper(II)–chitosan derivative and a strontium-substituted hydroxyapatite. These biomaterials showed ideal ion release profiles, offering burst release of an antibacterial agent together with a more sustained release of strontium in order to achieve long-term osteogenesis. We combined copper(II)–chitosan and strontium–hydroxyapatite into freeze-dried composite scaffolds. These scaffolds were characterized in terms of morphology, mechanical properties and bioactivity, defined here as the ability to trigger the deposition of novel calcium phosphate in contact with biological fluids. In addition, a preliminary biological characterization using cell line osteoblasts was performed. Our results highlighted that the combination of chitosan and hydroxyapatite in conjunction with copper and strontium has great potential in the design of novel scaffolds. Chitosan/HA composites can be an ideal technology for the development of tissue engineering scaffolds that deliver a complex arrays of therapeutic ions in both components of the composite, leading to tailored biological effects, from antibacterial activity, to osteogenesis and angiogenesis.
1 Introduction
Bone repair and regeneration constitute main challenges in reconstructive surgery as many conditions such as tumors, bone trauma, and bone infections cause critical bone defects. Regardless of the etiology, bone disorders are a major health concern with an expected cost by 2020 of $2.5 billion per year only in the USA.1 Major research efforts are being carried out worldwide in the field of allografts, xenografts and other artificial implants.2 However, to date, the golden standard in orthopedic surgery for the reconstruction and repair of bone defects still remains autologous bone.1–3 This consists of tissue harvested from a healthy anatomical site of the patient and transferred to the defect site. Autografts have two major drawbacks: limited availability and invasive harvesting, which can lead to inflammation, hematomas, donor site morbidity and even nerve damage.2 A possible strategy to avoid the drawbacks of autograft with the potential to revolutionize the current capability of medicine for bone repair and regeneration is bone tissue engineering (BTE).1 BTE combines cells, derived from a patient biopsy or from other sources (e.g. stem cells), with a scaffold, a three-dimensional porous structure made from specific biomaterials. Scaffolds are specifically designed to provide temporary support and able to stimulate cell growth and new tissue development,4 for example by delivery of biological signals, in most cases growth factors and biologically active molecules.3An effective BTE scaffold must own several key properties: interconnected porosity with adequate pore size distribution (10–400 μm), biocompatibility, biodegradability and/or bioresorbability over longer time periods, adequate mechanical properties in the short-term and physicochemical cues for the cells.4,5 The success of BTE scaffolds in clinical applications for bone repair and regeneration is linked to the fulfillment of this complex array of properties simultaneously, which implies that a composite approach, combining organic and inorganic biomaterials could be of great advantage.6 In fact, countless combinations of organic and inorganic phases are currently under study as composite scaffolds for BTE, exhibiting the unique feature of merging the properties of several materials in a single device. Comprehensive reviews on the topic are available.6–8 In this work the focus is on the use of two popular biomaterials in BTE approaches: chitosan as matrix phase and hydroxyapatite (HA) as filler phase.9
Hydroxyapatite (HA) has been widely studied and used for bone tissue engineering applications.10 The production of 3D HA scaffolds from precursor powders was demonstrated using several methods, including thermal bonding, phase leaching, foam replica and additive manufacturing.11 Inorganic HA scaffolds have optimal morphology and porosity, but are usually characterized by insufficient mechanical properties.11 For this reason, a composite approach aiming at the combination of HA powders with natural polymers was often preferred, proving to be a great candidate method for the development of scaffolds.9,12,13 Composite scaffolds based on HA powder dispersed in collagen, silk fibroin, gelatin and chitosan have been all extensively studied.14 Chitosan/HA scaffolds, in particular, are expected to present competitive biocompatibility, osteoconductivity and biodegradation together with sufficient mechanical strength for orthopedic use.9 HA (Ca10(PO4)6(OH)2) is a natural choice when designing novel bone TE approaches: it is a major component of vertebrate hard tissues and it makes up 60–70% of the mass of bone and 98% of the mass of dental enamel. Owing to its similarity to the natural mineral phase of human bone, artificial hydroxyapatite has been used considerably in biomedical applications for bone repair and bone regeneration, where osteoconductivity is considered a key property.10 Furthermore, the particular crystalline structure of hydroxyapatite allows the synthesis of HA derivatives in which ions in the lattice are substituted with other species of interest (i.e. therapeutic ions), making HA a versatile platform for the development of bioceramics with multifaceted biological properties, such as antibacterial effects, osteoinduction, angiogenesis regulation and others.15 These ions are naturally present in HA,16 but their content within the lattice can be artificially tailored and enhanced using several techniques, with specific focus on wet co-precipitation methods,16,17 as reviewed elsewhere.18 These methods offer high tailorability of the final composition of HA, with biologically relevant substitution of ions. For instance, desired ions can be added to a simulated body fluid (SBF) solution and then precipitated to obtain ion-substituted HA19 with biomimetic potential.20,21 Notably, the work published by Bonfield, Best and collaborators on the synthesis of silicon substituted HA showed improved attachment, growth and production of extracellular matrix by osteoblasts.22,23 Substituted HA has also already been combined with polymeric matrices in previous studies.24
This work focuses on the use of therapeutic metal ions (TMIs)25 as possible alternatives to the use of expensive biomolecules such as bone tissue growth factors (e.g. BMP-2). TMIs are a family of metal ions (e.g. strontium, zinc, magnesium or copper) that have been found to have a key role in the regulation of several physiological molecular pathways. TMIs are a main focus of current biomaterials research as they can positively interact with cells and provide a wide range of beneficial effects depending on the specific selected ion (angiogenesis, osteogenesis, improved proliferation or differentiation, antibacterial properties among others).25,26 Traditional approaches to use TMIs in biomaterials usually aim to combine the beneficial properties of ions embedded in particulate inorganic fillers together with a rather inert polymer matrix by means of composites fabrication. In this pilot study, a further improvement is proposed: the possibility to use TMI-carrying biomaterials in both phases of the composite is explored, effectively increasing the spectrum of properties that the final construct is able to deliver. In particular, the design and development of a chitosan/HA composite scaffold characterized by the delivery of strontium and copper ions by means of both its compositional phases is presented. Specifically, strontium is substituted in the HA lattice while copper is complexed with the chitosan matrix.
Strontium and copper were chosen as model ions to prove the concept of multiple ion delivery owing to their tremendously interesting beneficial properties. Strontium is the focus of a big portion of bone tissue engineering research because of its ability to enhance new bone formation and bone resorption.27 The effect of the addition of strontium on bone repair devices was investigated in previous studies and was linked to the ability of this metal ion to influence the gene expression of osteoclasts, shifting the balance of bone remodeling toward osteoblast-mediated bone formation and remodelling.27 Strontium can be delivered orally, but this mode lacks spatial control of the release.24 To locally stimulate regeneration/healing of a bone defect, strontium should be included directly in the scaffold. There are various materials science strategies for the local and controlled release of strontium in bone tissue engineering, many of which are already tested in vivo and in clinical trials, as systematically reviewed by Neves et al. in a recent publication.28 Investigations to date confirm the safety and efficacy of strontium-containing biomaterials and highlight a principal focus on the inclusion of the element in the structure (i.e. lattice/glass network) of inorganic biomaterials, mainly different forms of calcium phosphates (HA, β-TCP),29,30 silicate31,32 and borate28 bioactive glasses, or silicate bioceramics.31 Among other possible approaches, in this work the use of artificial hydroxyapatite was chosen due to the promising possibility to substitute calcium ions in the HA structure by strontium ions, even up to 100%.33
Copper was selected for its strong antibacterial properties and angiogenic effects.34,35 Copper ions are involved in the formation of reactive oxygen species (ROS) that can have dangerous detrimental effects if accumulated in excessive quantity. While eukaryotic cells have mechanisms to defend themselves from ROS, bacteria have weaker defenses and are quickly damaged. In parallel, copper competes for active sites of enzymes with other essential ions (e.g. calcium, iron), denaturing proteins and possibly damaging DNA.36 Conversely, copper can beneficially stimulate eukaryotic cells by inducing the overexpression of vascular endothelial growth factor (VEGF) and cycline D1 genes, stimulating angiogenesis and cell proliferation.37,38
In this study, we demonstrated for the first time the processability of copper(II)–chitosan into a porous copper-containing 3D matrix prepared by freeze-drying, optimizing previously published protocols.9 Strontium-substituted HA, a well established platform for the release of ions promoting bone formation and remodelling, was chosen as inorganic filler in order to enhance the native properties of the polymer. The presence of ions in the two biomaterials was confirmed and their release profiles were assessed. The fabrication of composite chitosan/HA scaffolds proved itself to be a versatile and reproducible way to prepare constructs with the unique property of dual and decoupled delivery of TMIs, loaded in both components of the composite scaffold. The scaffolds were characterized morphologically and physico-chemically using several techniques. Afterwards, a preliminary characterization of the cell response was also performed.
2 Materials and methods
2.1 Production of copper(II)–chitosan
2.2 Production of hydroxyapatite
2.3 Fabrication of HA–chitosan composite scaffolds
Freeze-drying with previously optimized parameters was performed for the production of HA–chitosan composite scaffolds.9 Copper(II)–chitosan with a 3% saturation of the amino groups (CuChi3, equivalent to 1 wt% copper) was chosen, as it guarantees the lowest cytotoxicity compared to other formulations.39 Chitosan or CuChi3 were dissolved in diluted acetic acid water solutions (3% v/v) at a concentration of 2% w/v. The solution was then cast in Eppendorf tubes used as negative molds and quickly frozen using liquid nitrogen (−196 °C). The rapid freezing encourages the formation of small and homogeneous water crystals, which will result in more reproducible morphologies. A design-of-experiment approach was adopted in order to screen the effect of the following variables on the properties of the scaffolds: (i) type of hydroxyapatite (either undoped or 3% strontium substituted), copper loading (from 0% to 3%) and filler-to-matrix ratio (33% to 80% wt). Table 1 summarizes the samples prepared in this study and the parameters used to produce them. The scaffolds are showed in Fig. 1. Cu1.5 samples were prepared mixing the same amount of undoped chitosan and CuChi3.| # | Sample name | Cu loading (wt%) | Type of HA | Filler-to-matrix ratio (%) |
|---|---|---|---|---|
| 1 | Cu0 HA33 | 0 | Undoped | 33 |
| 2 | Cu0 HA80 | 0 | Undoped | 80 |
| 3 | Cu3 HA33 | 3 | Undoped | 33 |
| 4 | Cu3 HA80 | 3 | Undoped | 80 |
| 5 | Cu0 SrHA33 | 0 | 3% strontium | 33 |
| 6 | Cu0 SrHA80 | 0 | 3% strontium | 80 |
| 7 | Cu3 SrHA33 | 3 | 3% strontium | 33 |
| 8 | Cu3 SrHA80 | 3 | 3% strontium | 80 |
| 9 | Cu1.5 HA56.5 | 1.5 | Undoped | 56.5 |
| 10 | Cu1.5 SrHA56.5 | 1.5 | 3% strontium | 56.5 |
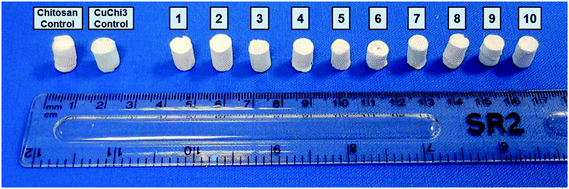 | ||
| Fig. 1 Samples of all ten formulations of the composite scaffolds produced by freeze-drying in this study. | ||
2.4 Physical characterization of the scaffolds
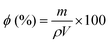 | (1) |
2.5 Biological characterization of the scaffolds
The deposition of novel HCA was qualitatively assessed by SEM using the same set-up previously described. Fourier transform infrared spectroscopy (FTIR) using a Shimadzu IRAffinity-1S (Shimadzu Corp, Japan) equipped with a Quest ATR GS10801-B single bounce diamond accessory (Specac Ltd, England) was also performed. Data were acquired with 40 scans at a resolution of 4 cm−1 and processed using the LabSolution IR software by Shimadzu. Native chitosan scaffolds were used as controls in both analyses.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio and the cell suspension was counted by trypan blue exclusion method (Sigma-Aldrich, Germany). Cells were seeded in 24-well culture plates at a density of 100
3 ratio and the cell suspension was counted by trypan blue exclusion method (Sigma-Aldrich, Germany). Cells were seeded in 24-well culture plates at a density of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and incubated overnight at 37 °C, 95% relative humidity and 5% CO2. In parallel, samples of all ten scaffold varieties were UV-sterilized for one hour and preconditioned in DMEM for 24 hours. Control samples of native chitosan and copper(II)–chitosan without filler were also similarly prepared. On the second day, the samples were added to the wells of cell culture and incubated for another 24 hours. Finally, the samples were removed and cell viability and fluorescent staining were performed.
000 cells per well and incubated overnight at 37 °C, 95% relative humidity and 5% CO2. In parallel, samples of all ten scaffold varieties were UV-sterilized for one hour and preconditioned in DMEM for 24 hours. Control samples of native chitosan and copper(II)–chitosan without filler were also similarly prepared. On the second day, the samples were added to the wells of cell culture and incubated for another 24 hours. Finally, the samples were removed and cell viability and fluorescent staining were performed.
 | (2) |
3 Results
3.1 Copper complexation and release from copper(II)–chitosan
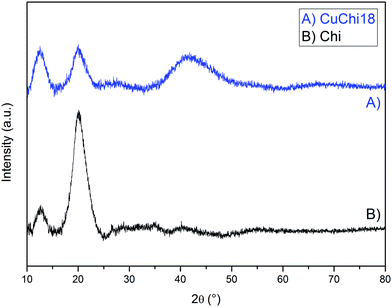 | ||
| Fig. 2 Comparison between XRD spectra of native chitosan and CuChi18. All other copper(II)–chitosan formulations behave similarly. | ||
The diffractogram of native chitosan showed two broad diffraction peaks at 2θ = 12° and 20°, which are characteristic fingerprints of semi-crystalline chitosan from the selected supplier, as previously reported.46 These peaks are associated to the formation of crystal I and crystal II chitosan structures. After the addition of copper, a decrease in the crystal II peak at 2θ = 20° was observed for all tested formulations, without significant differences between formulations with different levels of copper. No previous literature was found regarding the broad shoulder found at 2θ = 40°. This could be ascribed to the formation of a new crystal conformation of chitosan after the chelation or simply to an increased signal from amorphous domains of the polysaccharide. Further crystallographic studies on the topic should be performed in order to clarify this finding.
| Cumulative amount of copper released (ppm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CuChi3 | CuChi6 | CuChi12 | CuChi15 | ||||||||||
| Days | 1 | 18 | 20 | 20 | 33 | 35 | 34 | 70 | 70 | 68 | 85 | 83 | 87 |
| 7 | 19 | 20 | 20 | 35 | 34 | 34 | 70 | 70 | 69 | 88 | 86 | 84 | |
| 14 | 19 | 21 | 19 | 36 | 34 | 36 | 70 | 71 | 70 | 88 | 87 | 88 | |
3.2 Strontium substitution and release from hydroxyapatite
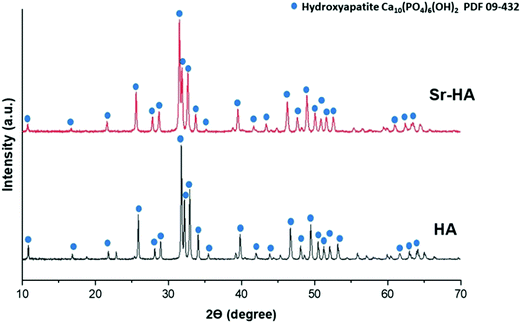 | ||
| Fig. 4 X-ray diffractograms of hydroxyapatite (HA) and strontium substituted hydroxyapatite (Sr–HA). | ||
| a-Axis | c-Axis | |
|---|---|---|
| HA | 9.4251 | 6.8845 |
| Sr–HA | 9.4845 | 6.9481 |
| Samples | Calcium (mol%) | Phosphorus (mol%) | Strontium (mol%) | Ca + Sr/P ratio | |
|---|---|---|---|---|---|
| Calculated | XRF | ||||
| HA | 1.07 | 0.6481 | — | 1.667 | 1.6509 |
| Sr–HA | 0.8649 | 0.5917 | 0.1254 | 1.667 | 1.6736 |
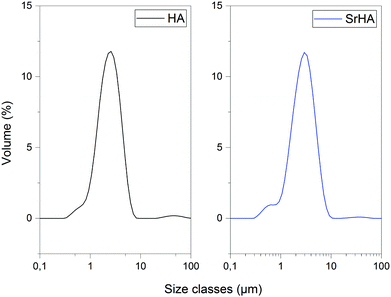
| Hydroxyapatite | Strontium substituted hydroxyapatite | |
|---|---|---|
| Dx (10) | 1.25 | 1.32 |
| Dx (50) | 2.41 | 2.78 |
| Dx (90) | 4.37 | 5.13 |
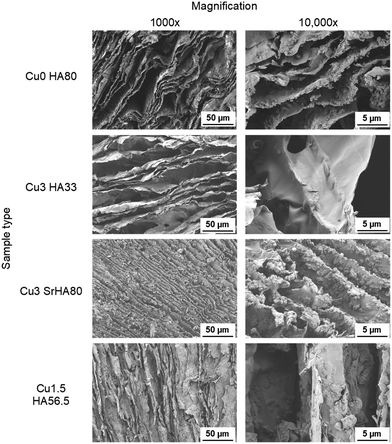
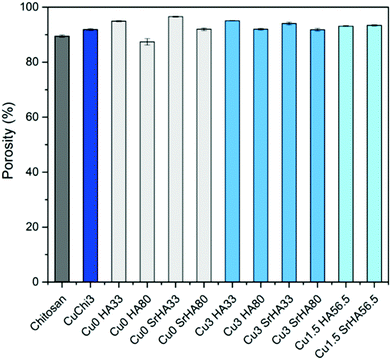
3.3 Characterization of scaffold morphology and porosity
3.4 Biological characterization
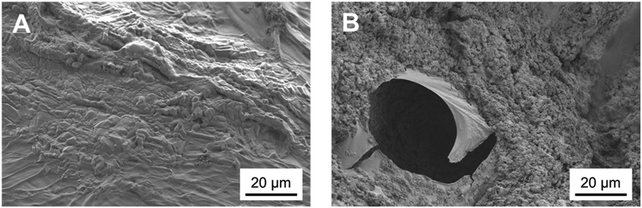
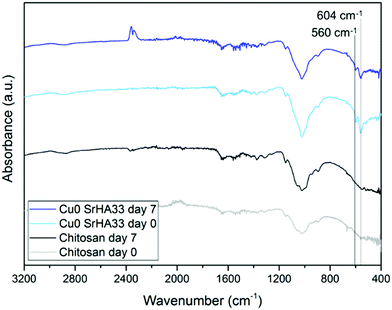
The nature of this layer was unclear from the SEM imaging: the shape of the crystal suggested the formation of HCA, but the cauliflower structure typical of HCA grown in SBF could not be detected.43 This suggests that this layer could be a deposit of amorphous calcium phosphates, precursors of HCA.42 The spectroscopic analysis was not effective in the identification of the nature of this layer as FTIR spectra before and after immersion did not show significant variations. This occurred because specimens were either not bioactive (that is the case of the control samples of chitosan and CuChi3) or already rich in HA. The presence of hydroxyapatite prior to testing, in the case of the composite samples, resulted in the shielding of possible variations in characteristic peaks of the P–O bending at 560 and 604 cm−1 as a consequence of the immersion in SBF.45 As it can be observed in Fig. 12, the spectra of chitosan control and Cu0SrHA33 before and after testing do not show significant alterations.
Altogether the results of the acellular bioactivity, or apatite-forming ability, test suggest the deposition of a calcium phosphate layer absent on control samples: HA has a key role in making the scaffolds bioactive; however, further studies will be performed in order to provide conclusive evidence on the nature of the deposit detected in this study. For example, a novel SBF using easily detectable 44Ca isotope of calcium could be a way to better describe the results of the immersion. Otherwise, Raman spectroscopy due to its higher sensitivity could be to accurately identify newly formed hydroxyl-carbonate apatite.
4 Discussion
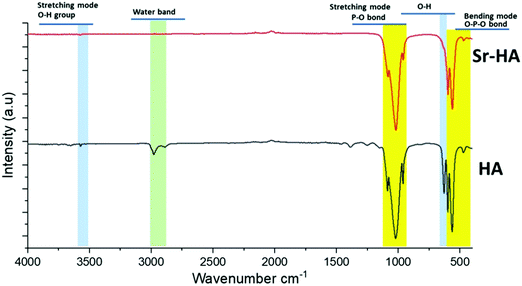
Copper(II)–chitosan was prepared following the protocol introduced in our previous publication.39 The presence of copper and successful complexation with chitosan were previously confirmed by EDX and FTIR.39 In this work an analysis of the material by XRD was also performed, showing the presence of significant variations in the diffractogram of chitosan following the addition of copper. In particular, the complexation of copper induces a significant decrease in the 2θ = 20° peak of the diffractogram, which is characteristic for the crystal II structure of chitosan.46 This variation was previously reported for a complex of chitosan and iron: the authors suggested that this change in the diffractogram could be ascribed to a decrease in crystallinity of the final material compared to native chitosan.46 The complexation of chitosan with transition metals creates a reduction in binding sites available for hydrogen bonding (NH2 and OH), decreasing the number of inter- and intramolecular bonds between chitosan chains necessary for self-assembly of the polysaccharide.In parallel, a wet precipitation method was used to successfully produce artificial HA and strontium-substituted HA powders. XRD results showed typical peaks of the hexagonal structure of HA, confirming the high crystallinity of these two materials. Only minor variations were identified as a consequence of the ion substitution and the expansion in the HA unit cell.50 In particular, Sr–HA samples were characterized by general peak broadening and shifts in low 2θ angle peaks, which are typical evidences of ion substitution.49 The analysis by FTIR further confirmed the findings of the XRD analyses: typical peaks and bands of phosphate and hydroxyl vibrations were detected both in HA and Sr–HA. In addition, a minor signal that can be ascribed to carbonate impurities was also identified. Finally, XRF was fundamental in confirming that the calcium phosphate produced was actually hydroxyapatite and to quantify the amount of strontium substituted. In previous studies, authors suggested that XRF is an effective technique for the quantification of substitutions in HA. However, other relevant techniques to perform this measurement could be ICP-MS or atomic absorption spectroscopy (AAS).21 The calculation of the Ca/P molar ratio performed from the results of compositional analysis confirmed a value very close to that characteristic of stoichiometric HA (1.667). These results are in good agreement with previously reported results in literature.51,55–57 Li et al.51 reported that Sr–HA substituted with 0.3 mol% and 1.5 mol% Sr2+ ions showed a monophasic spectrum comparable to pure HA. Kim et al.55 revealed that substitution of Sr2+ over 4 mol% caused transformation in the microstructure from sphere-shaped grains to faceted grains, also β-TCP was formed as secondary phase. The studies by Shen et al.57 about the effect of Sr2+ substitution on compressive strength of calcium phosphate cements (CPC) indicated that addition of 5 wt% Sr–HA whisker in CPC induced higher compressive strength of 2.91 MPa, which is approximately double than that of pure CPC. Kim et al.55 proved that 8 mol% substitution of Sr2+ in Sr–HA caused the increase in Vicker's hardness from 5.2 to 5.5 GPa. Capuccini et al.56 carried out in vitro tests using osteoblast-like MG-63 and human osteoclasts cells. Their results revealed that Sr–HA substituted with 3–7 at% Sr2+ ions considerably stimulated osteoblast proliferation and differentiation, also they reported that 1 at% substitution of Sr2+ ions was significant to reduce the osteoclast proliferation.56
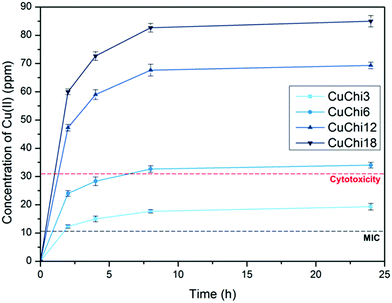
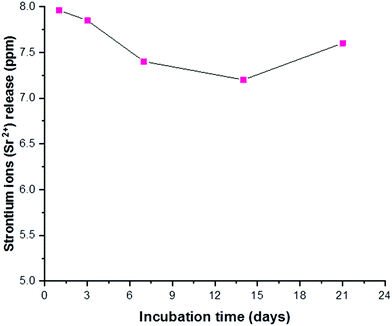
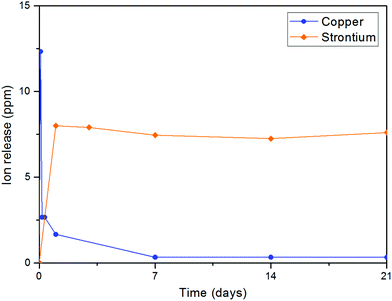
Before using the two synthesized biomaterials for the fabrication of composite scaffolds, ion release tests were also performed. Copper was quantified by a cost-effective capillary electrophoresis method previously developed.40 Results confirmed that copper(II)–chitosan is characterized by a burst release of copper within the first ten hours. The release can be finely tuned by adjusting the amount of copper initially loaded in the polysaccharide. In particular, the formulation CuChi3, used for the production of the scaffolds, releases around 15–20 ppm of copper within the first few hours; this is an ideal release, which can inhibit the colonization by bacteria upon implantation, without excessive cytotoxic effects. It is important to notice that after the burst release a significant quantity of copper is still embedded in the chitosan and will be released only upon degradation of the polymer, which does not occur in saline solutions (i.e. PBS).58 Ion release tests after enzymatic degradation should be performed in order to quantify longer term release of copper from copper(II)–chitosan, which could still be helpful in impeding later stage bacterial colonization and enhancing angiogenesis.38 The release of strontium from Sr–HA was simultaneously determined by ICP-OES and follows a very different trend compared to the one of copper: release reached equilibrium at around 8 ppm per each time point. Strontium release at this concentration sits within the therapeutic window (2–45 ppm) and it is expected to help osteogenesis throughout the first months of regeneration in vivo.59 The different nature of the TMI loading in the two materials highlights the convenience of the present strategy to obtain very different release profiles depending on the desired ion, tailoring the uniformity of release according to need independently of the intrinsic scaffold degradation kinetics (Fig. 15).
After the characterization of the biomaterials and the investigation of ion release, copper(II)–chitosan and strontium substituted hydroxyapatite were successfully combined and fabricated into composite scaffolds for bone tissue engineering by liquid nitrogen freeze-drying, using chitosan as the matrix and HA as the particulate filler phase. In comparison to previously reported systems, in this work a novel concept for the preparation of bone TE scaffolds is introduced, which offers the possibility to dope both the matrix and the filler of the composite with TMIs. In this work, copper and strontium were used. However, chitosan and hydroxyapatite are very versatile material platforms that could allow the design of more complex arrays of ion doping and release. At the same time, the fabrication of TE scaffolds by freeze-drying as reported in this work can be adapted to other derivatives of chitosan and HA. This allows the production of constructs able to deliver several ions with different release profile in time, depending on the specific need. In fact, different ions are needed at different times in order to effectively guide cell adhesion and proliferation as well as, at a later stage, tissue ingrowth and vascularization.25 Having the possibility to engineer the release of TMIs to follow these complex profiles can allow precise engineering of the several stages of inflammation, healing and tissue regeneration. In parallel, TMIs offer also the advantage of enhancing the antibacterial properties of scaffolds with little risk of resistance development. In fact, the antibacterial activity of TMIs is known to be quick and multifaceted: such rapid action inhibits the intrinsic ability of bacteria to develop resistance.60
Liquid nitrogen freeze-drying is a robust and reproducible fabrication technique, especially in terms of morphology and porosity. Chitosan/HA composite scaffolds prepared in this study are characterized by tailorable, hierarchical and interconnected porosity in the ideal range for cell infiltration and correct differentiation.54 The macroscale pore size and distribution can be finely tailored through the variation of the processing parameters, especially by controlling the freezing process and the composition of the HA/chitosan suspensions.9 In particular, rapid freezing with liquid nitrogen is an effective way to decreases the average size of the water crystal formed and thus of the pores. In comparison with previously reported chitosan-based scaffolds,61–63 this approach is an important improvement in order to achieve better control of scaffold morphology. Indeed, in previously developed protocols for the preparation of chitosan/hydroxyapatite scaffolds nitrogen freezing has rarely been used.61–63 Madihally et al.,64 for instance, proposed to use liquid nitrogen for chitosan scaffolds as a solution to achieve pore size and morphology control and bring them closer to physiologically relevant levels. Our results confirm this hypothesis, as chitosan/HA scaffolds prepared in this work have a fairly homogeneous distribution of interconnected pores with a size in the range of 10–100 μm,65 which is known to be ideal for cell infiltration.54,66 Tailoring pore morphology is a key challenge to correctly regulate cell colonization rates and engineer the organization of new tissue into scaffolds.54 In addition to this, pore size, morphology and interconnectivity are known to be key variables in controlling degradation kinetics, mechanical properties and angiogenesis.4,64,67
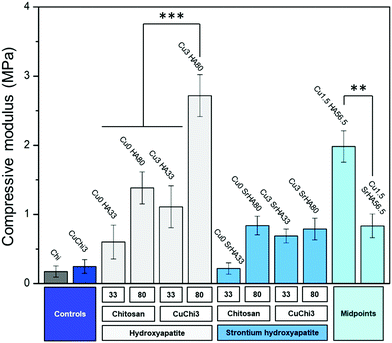
The introduction of hydroxyapatite in the chitosan matrix was expected to give the composite several beneficial properties that native chitosan lacks. In terms of mechanical properties, the inorganic filler is a key factor in increasing the compressive modulus of the scaffolds. This finding is in agreement with previously reported composite scaffolds and in general with theories on composite materials.9,68 In particular, the scaffold formulations that showed the best mechanical performance are Cu0HA80 and Cu1.5HA56.5. These samples reached values of compressive modulus of around 2–3 MPa, which are approaching the lower end of the modulus of trabecular bone,67 but should be further improved. Interestingly, HA was found to perform better than Sr–HA. Strontium-substituted hydroxyapatite resulted in moduli which barely reached 1 MPa even for high filler loadings. An explanation for this behavior could be a consequence of poorer interface properties: possible leaching of strontium ions from the HA, even in trace amounts, could have been absorbed by the chitosan matrix, inducing the formation of complexes at the interface and resulting in embrittlement and reduction in compliance.69 This local cross-linking of chitosan as a consequence of the complexation could have determined a reduction in the ability of chitosan to deform under compression, increasing the formation of interface voids. In future, interfacial phenomena between Sr–HA and chitosan should be characterized and the interface itself improved by, for example, surface modification of the HA filler. In addition to improving mechanical properties, HA and Sr–HA containing scaffolds were effective in triggering the deposition of a calcium phosphate layer after 7 days in SBF. In contrast, no deposition was assessed on control scaffolds (i.e. chitosan and copper(II)–chitosan). In these samples, the only consequence of the immersion in SBF is the collapse of most of the porosity upon dehydration, which highlights the poor integrity of native chitosan scaffolds and the need for a reinforcing filler to increase mechanical properties, as previously discussed. However, shielding of the signal of this layer by HA initially present in the scaffolds made FTIR analysis inconclusive. Longer incubation times (e.g. 21 or 28 days) in SBF would probably show clearer results. Avoiding the use of Tris buffer is also an important aspect for future optimization of the test: the addition of this buffer in SBF was in fact linked to a possible distortion in the apatite-forming ability of bioactive materials.28 Tris seems to increase the dissolution rate of bioactive glasses (particularly Bioglass® 45S5) and therefore overexpress their bioactivity. Although HA is more stable and less susceptible to possible chemical attack of Tris,28 the possible occurrence of this phenomenon should be verified. Further investigation is needed to clarify the actual extent of scaffold bioactivity and the nature of the deposited phase.
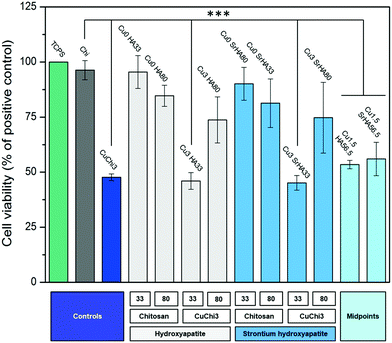
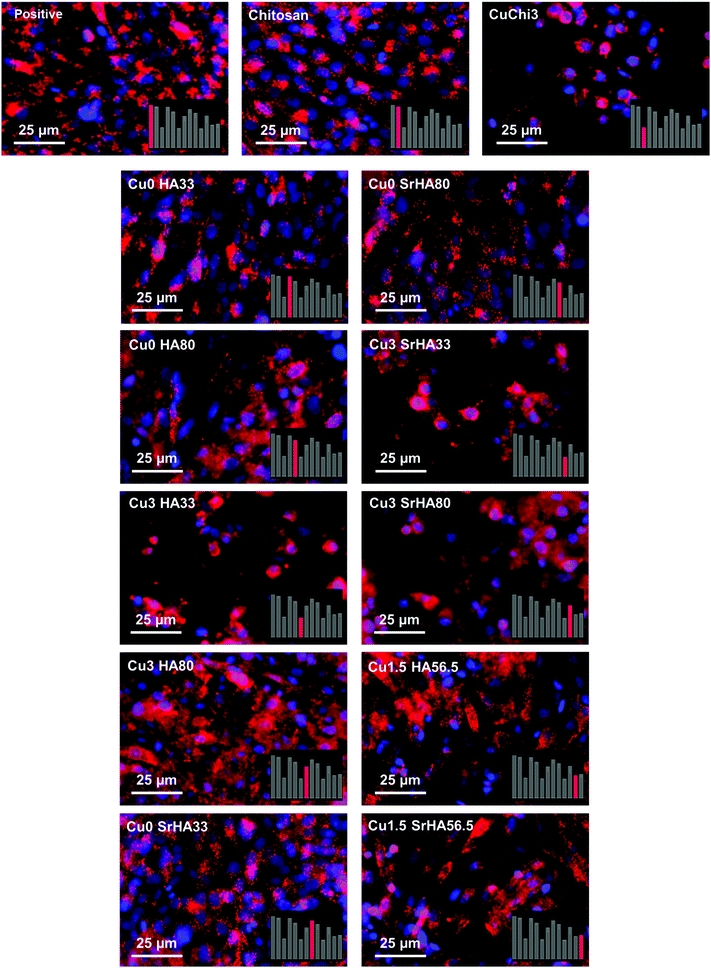
Finally, a preliminary cell culture study was performed using the MG-63 human osteoblast-like cell line. The results of cell viability, measured by WST-8, showed that the ions release had mildly cytotoxic effects only in those samples with little to no HA phase. Fluorescence images of cell morphology are in agreement with cell viability results. In particular, it appears that the presence of HA reduces the cytotoxic character of copper. This is likely due to a protective effect of ions leaching from hydroxyapatite, which can establish biomolecular competition for copper-binding sites.70,71 The lower viability of the CuChi3 sample is somehow in contrast with previous results on the same material39 and could be due to the increase in surface area of the porous samples compared to bulk ones, highlighting the need to perform ion release tests on the full scaffolds.72 These preliminary findings foster further studies to optimize and expand the possible fabrication routes of scaffolds loaded with multiple ions and to characterize the physicochemical and biological performance of these promising tissue engineering platforms. Specifically, in-depth investigations should be carried out in order to explore more combinations of ions and to unravel the specific responses that these ions elicit on cells when released simultaneously and in a controlled manner.
5 Concluding remarks
Two candidate platforms for the delivery of TMIs were prepared: chitosan complexed with copper and strontium substituted hydroxyapatite. The effective loading and release of ions from the two materials was characterized and their use in the production of novel bone TE composite scaffolds with dual ion delivery was demonstrated. The release of copper and strontium follows significantly different profiles due to the different nature of the loading. In particular, copper experiences a burst release from chitosan allowing it to quickly stop possible bacterial colonization, while strontium is released uniformly in order to help and support the regeneration process over a longer period. The materials can be prepared into scaffolds using an optimized and reproducible freeze drying protocol, using chitosan as matrix and a fine, homogeneous particulate HA phase as filler. The resulting scaffolds are ideal for cell infiltration, cell migration and neovascularization thanks to their reproducible pore morphology. They are characterized by interconnected porosity and increased mechanical properties depending on the filler content. Cell cultures performed on the composite scaffolds showed that the materials are also cytocompatible. Compared to existing technologies, chitosan/HA scaffolds produced in this study exhibit the novel feature of carrying therapeutic ions both in the matrix and the filler of the composite. In this study, copper and strontium were loaded respectively in chitosan and hydroxyapatite. However, these two biomaterials are highly versatile and can chelate (chitosan) and include (hydroxyapatite) several different ions in their structure. The combination of chitosan and hydroxyapatite has the potential to design scaffolds with the capability to deliver TMIs depending on the specific need in terms of tissue regeneration properties. The two materials are versatile carriers of biologically relevant ions. In the future, the possibility to have more complex arrays of therapeutic ions in both components of the composite, targeting different cells and tissues, should be explored.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie (HyMedPoly project, Grant Agreement No. 643050). We acknowledge the important support of the whole HyMedPoly consortium. We would also like to thank Mr Mike Salt for its technical guidance with freeze-drying and of Mr Lee Bullock for mechanical testing consultancy.References
- A. R. Amini, C. T. Laurencin and S. P. Nukavarapu, CRC Crit. Rev. Bioeng., 2012, 40, 363–408 Search PubMed.
- R. A. Bhatt and T. D. Rozental, Handb. Clin. Neurol., 2012, 28, 457–468 Search PubMed.
- M. Jafari, Z. Paknejad, M. R. Rad, S. R. Motamedian, M. J. Eghbal, N. Nadjmi and A. Khojasteh, J. Biomed. Mater. Res., Part B, 2017, 105, 431–459 CrossRef CAS PubMed.
- F. J. O’Brien, Mater. Today, 2011, 14, 88–95 CrossRef.
- L. Gritsch, D. Meng and A. R. Boccaccini, Biomed. Compos., 2017, 501–542 Search PubMed.
- K. Rezwan, Q. Z. Chen, J. J. Blaker and A. R. Boccaccini, Biomaterials, 2006, 27, 3413–3431 CrossRef CAS PubMed.
- N. Goonoo, A. Bhaw-Luximon, P. Passanha, S. R. Esteves and D. Jhurry, J. Biomed. Mater. Res., Part B, 2017, 105, 1667–1684 CrossRef CAS PubMed.
- A. R. Boccaccini, M. Erol, W. J. Stark, D. Mohn, Z. Hong and J. F. Mano, Compos. Sci. Technol., 2010, 70, 1764–1776 CrossRef CAS.
- L. Pighinelli and M. Kucharska, Carbohydr. Polym., 2013, 93, 256–262 CrossRef CAS PubMed.
- F. Baino, G. Novajra and C. Vitale-Brovarone, Front. Bioeng. Biotechnol., 2015, 3, 202 Search PubMed.
- S. Deville, E. Saiz, R. K. Nalla and A. P. Tomsia, Adv. Sci. Technol., 2006, 49, 148–152 CAS.
- V. Brun, C. Guillaume, S. Mechiche Alami, J. Josse, J. Jing, F. Draux, S. Bouthors, D. Laurent-Maquin, S. C. Gangloff, H. Kerdjoudj and F. Velard, Biomed. Mater. Eng., 2014, 24, 63–73 CAS.
- H. Li, C. Hu, H. Yu and C. Chen, RSC Adv., 2018, 8, 3736–3749 RSC.
- H. Siddiqui, K. Pickering and M. Mucalo, Materials, 2018, 11, 1813 CrossRef PubMed.
- J. T. B. Ratnayake, M. Mucalo and G. J. Dias, J. Biomed. Mater. Res., Part B, 2017, 105, 1285–1299 CrossRef CAS PubMed.
- I. Cacciotti, Handbook of Bioceramics and Biocomposites, 2016, pp. 145–211 Search PubMed.
- S. Sprio, A. Tampieri, E. Landi, M. Sandri, S. Martorana, G. Celotti and G. Logroscino, Mater. Sci. Eng., C, 2008, 28, 179–187 CrossRef CAS.
- T. Tite, A. C. Popa, L. M. Balescu, I. M. Bogdan, I. Pasuk, J. M. F. Ferreira and G. E. Stan, Materials, 2018, 11, E2081 CrossRef PubMed.
- A. C. Tas, J. Eur. Ceram. Soc., 2000, 20, 2389–2394 CrossRef CAS.
- M. Šupová, Ceram. Int., 2015, 41, 9203–9231 CrossRef.
- G. Graziani, M. Boi and M. Bianchi, Coatings, 2018, 8, 269 CrossRef.
- E. S. Thian, J. Huang, M. E. Vickers, S. M. Best, Z. H. Barber and W. Bonfield, J. Mater. Sci., 2006, 41, 709–717 CrossRef CAS.
- I. R. Gibson, S. M. Best and W. Bonfield, J. Biomed. Mater. Res., 1999, 44, 422–428 CrossRef CAS PubMed.
- Y. Lei, Z. Xu, Q. Ke, W. Yin, Y. Chen, C. Zhang and Y. Guo, Mater. Sci. Eng., C, 2017, 72, 134–142 CrossRef CAS PubMed.
- V. Mouriño, J. P. Cattalini and A. R. Boccaccini, J. R. Soc., Interface, 2012, 9, 401–419 CrossRef PubMed.
- A. Philippart, N. Gómez-Cerezo, D. Arcos, A. J. Salinas, E. Boccardi, M. Vallet-Regi and A. R. Boccaccini, J. Non-Cryst. Solids, 2017, 455, 90–97 CrossRef CAS.
- P. J. Marie, P. Ammann, G. Boivin and C. Rey, Calcif. Tissue Int., 2001, 69, 121–129 CrossRef CAS PubMed.
- Y. Zhang, X. Cui, S. Zhao, H. Wang, M. N. Rahaman, Z. Liu, W. Huang and C. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 2393–2403 CrossRef CAS PubMed.
- U. Thormann, S. Ray, U. Sommer, T. ElKhassawna, T. Rehling, M. Hundgeburth, A. Henß, M. Rohnke, J. Janek, K. S. Lips, C. Heiss, G. Schlewitz, G. Szalay, M. Schumacher, M. Gelinsky, R. Schnettler and V. Alt, Biomaterials, 2013, 34, 8589–8598 CrossRef CAS PubMed.
- G.-M. Kuang, W. P. Yau, J. Wu, K. W. K. Yeung, H. Pan, W. M. Lam, W. W. Lu and K. Y. Chiu, J. Biomed. Mater. Res., Part A, 2015, 103, 1613–1621 CrossRef PubMed.
- A. A. Gorustovich, T. Steimetz, R. L. Cabrini and J. M. P. López, J. Biomed. Mater. Res., Part A, 2010, 92A, 232–237 CrossRef CAS PubMed.
- G. Molino, A. Bari, F. Baino, S. Fiorilli and C. Vitale-Brovarone, J. Mater. Sci., 2017, 52, 9103–9114 CrossRef CAS.
- R. Z. LeGeros, Clinical Orthopaedics and Related Research, 2002, pp. 81–98 Search PubMed.
- H. Xie and Y. J. Kang, Curr. Med. Chem., 2009, 16, 1304–1314 CrossRef CAS PubMed.
- G. Grass, C. Rensing and M. Solioz, Appl. Environ. Microbiol., 2011, 77, 1541–1547 CrossRef CAS PubMed.
- R. B. Thurman and C. P. Gerba, Crit. Rev. Environ. Control, 1989, 18, 298–315 Search PubMed.
- M. L. Turski and D. J. Thiele, J. Biol. Chem., 2009, 284, 717–721 CrossRef CAS PubMed.
- L. D. D’Andrea, A. Romanelli, R. Di Stasi and C. Pedone, Dalton Trans., 2010, 39, 7625 RSC.
- L. Gritsch, C. Lovell, W. H. Goldmann and A. R. Boccaccini, Carbohydr. Polym., 2018, 179, 370–378 CrossRef CAS PubMed.
- J. P. Cattalini, V. S. Mouriño and S. E. Lucangioli, Anal. Methods, 2016, 8, 7767–7773 RSC.
- M. R. Saeri, A. Afshar, M. Ghorbani, N. Ehsani and C. C. Sorrell, Mater. Lett., 2003, 57, 4064–4069 CrossRef CAS.
- T. Kokubo and H. Takadama, Biomaterials, 2006, 27, 2907–2915 CrossRef CAS PubMed.
- E. Boccardi, A. Philippart, V. Melli, L. Altomare, L. De Nardo, G. Novajra, C. Vitale-Brovarone, T. Fey and A. R. Boccaccini, Ann. Biomed. Eng., 2016, 44, 1881–1893 CrossRef CAS PubMed.
- A. L. B. Maçon, T. B. Kim, E. M. Valliant, K. Goetschius, R. K. Brow, D. E. Day, A. Hoppe, A. R. Boccaccini, I. Y. Kim, C. Ohtsuki, T. Kokubo, A. Osaka, M. Vallet-Regí, D. Arcos, L. Fraile, A. J. Salinas, A. V. Teixeira, Y. Vueva, R. M. Almeida, M. Miola, C. Vitale-Brovarone, E. Verné, W. Höland and J. R. Jones, J. Mater. Sci.: Mater. Med., 2015, 26, 1–10 CrossRef PubMed.
- F. Ciraldo, L. Liverani, L. Gritsch, W. Goldmann and A. Boccaccini, Materials, 2018, 11, 692 Search PubMed.
- J. Qu, Q. Hu, K. Shen, K. Zhang, Y. Li, H. Li, Q. Zhang, J. Wang and W. Quan, Carbohydr. Res., 2011, 346, 822–827 CrossRef CAS PubMed.
- M. C. Moolman, Z. Huang, S. T. Krishnan, J. W. J. Kerssemakers and N. H. Dekker, J. Nanobiotechnol., 2013, 11, 12 CrossRef PubMed.
- M. J. Woźniak-Budych, K. Langer, B. Peplińska, Ł. Przysiecka, M. Jarek, M. Jarzębski and S. Jurga, Mater. Chem. Phys., 2016, 179, 242–253 CrossRef.
- K. Ozeki, T. Goto, H. Aoki and T. Masuzawa, Bio-Med. Mater. Eng., 2014, 24, 1447–1456 CAS.
- W. Zhang, N. Cao, Y. Chai, X. Xu and Y. Wang, Ceram. Int., 2014, 40, 16061–16064 CrossRef CAS.
- Z. Y. Li, W. M. Lam, C. Yang, B. Xu, G. X. Ni, S. A. Abbah, K. M. C. Cheung, K. D. K. Luk and W. W. Lu, Biomaterials, 2007, 28, 1452–1460 CrossRef CAS PubMed.
- C. M. Mardziah, I. Sopyan, M. Hamdi and S. Ramesh, Med. J. Malays., 2008, 63(suppl. A), 79–80 Search PubMed.
- Z. H. Cheng, A. Yasukawa, K. Kandori and T. Ishikawa, Langmuir, 1998, 14, 6681–6686 CrossRef CAS.
- Q. L. Loh and C. Choong, Tissue Eng., Part B, 2013, 19, 485–502 CrossRef CAS PubMed.
- H. W. Kim, Y. H. Koh, Y. M. Kong, J. G. Kang and H. E. Kim, J. Mater. Sci.: Mater. Med., 2004, 15, 1129–1134 CrossRef CAS PubMed.
- C. Capuccini, P. Torricelli, E. Boanini, M. Gazzano, R. Giardino and A. Bigi, J. Biomed. Mater. Res., Part A, 2009, 89, 594–600 CrossRef CAS PubMed.
- Y. Shen, J. Liu, K. Lin and W. Zhang, Mater. Lett., 2012, 70, 76–79 CrossRef CAS.
- M. Periayah, A. Halim and A. M. Saad, Pharmacogn. Rev., 2016, 10, 39 CrossRef CAS PubMed.
- A. Aimaiti, A. Maimaitiyiming, X. Boyong, K. Aji, C. Li and L. Cui, Stem Cell Res. Ther., 2017, 8, 282 CrossRef PubMed.
- C. Walsh, Nature, 2000, 406, 775–781 CrossRef CAS PubMed.
- I. Adekogbe and A. Ghanem, Biomaterials, 2005, 26, 7241–7250 CrossRef CAS PubMed.
- T. Garg, A. Chanana, R. Joshi and S. G. L. Bihani, IOSR J. Pharm., 2012, 2, 72–73 Search PubMed.
- G. Pereira, M. Amaral, P. Cesar, M. Bottino, C. Kleverlaan and L. Valandro, J. Mech. Behav. Biomed. Mater., 2015, 45, 183–192 CrossRef CAS PubMed.
- S. V. Madihally and H. W. T. Matthew, Biomaterials, 1999, 20, 1133–1142 CrossRef CAS PubMed.
- D. Narayan and S. S. Venkatraman, J. Biomed. Mater. Res., Part A, 2008, 87, 710–718 CrossRef CAS PubMed.
- L. Gritsch, D. Meng and A. R. R. Boccaccini, Biomedical Composites, Elsevier, 2017, pp. 501–542 Search PubMed.
- E. Boccardi, F. E. Ciraldo and A. R. Boccaccini, MRS Bull., 2017, 42, 226–232 CrossRef CAS.
- Y. Y. Zheng, Y. Y. Zheng and R. Ning, Mater. Lett., 2003, 57, 2940–2944 CrossRef CAS.
- L. Lupa, R. Voda and A. Popa, Sep. Sci. Technol., 2018, 53, 1107–1115 CrossRef CAS.
- T. Dudev and C. Lim, Chem. Rev., 2014, 114, 538–556 CrossRef CAS PubMed.
- T. Yamazaki, M. Kobayashi, K. Hirano, H. Onuki, J. Shimada, A. Yamazaki, Y. Hibino, H. Nakajima, Y. Yokote, S. Takemoto, Y. Oda and H. Sakagami, In Vivo, 2012, 26, 651–656 CAS.
- M. Manzano and M. Vallet-Regí, J. Mater. Chem., 2010, 20, 5593–5604 RSC.
| This journal is © The Royal Society of Chemistry 2019 |