Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-crosslinkable recombinant collagen mimics for tissue engineering applications
Liesbeth
Tytgat
ab,
Marica
Markovic†
c,
Taimoor H.
Qazi
 d,
Maxime
Vagenende
ab,
Fabrice
Bray
d,
Maxime
Vagenende
ab,
Fabrice
Bray
 e,
José C.
Martins
e,
José C.
Martins
 f,
Christian
Rolando
f,
Christian
Rolando
 e,
Hugo
Thienpont
a,
Heidi
Ottevaere
e,
Hugo
Thienpont
a,
Heidi
Ottevaere
 a,
Aleksandr
Ovsianikov†
a,
Aleksandr
Ovsianikov†
 c,
Peter
Dubruel
c,
Peter
Dubruel
 b and
Sandra
Van Vlierberghe
b and
Sandra
Van Vlierberghe
 *ab
*ab
aBrussels Photonics (B-PHOT) – Department of Applied Physics and Photonics, Vrije Universiteit Brussel, Pleinlaan 2, 1050 Brussels, Belgium
bPolymer Chemistry & Biomaterials Group – Centre of Macromolecular Chemistry (CMaC) – Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281, S4-Bis, 9000 Ghent, Belgium. E-mail: sandra.vanvlierberghe@ugent.be
cInstitute of Materials Science and Technology, TU Wien, Getreidemarkt 9, 1060 Vienna, Austria
dJulius Wolff Institute, Charité – Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany
eMiniaturisation pour l’Analyse, la Synthèse et la Protéomique, USR 3290 Centre National de la Recherche Scientifique, University of Lille, Villeneuve d’Ascq, France
fNMR and Structure Analysis Unit – Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281, S4-Bis, 9000 Ghent, Belgium
First published on 15th April 2019
Abstract
Gelatin is frequently used in various biomedical applications. However, gelatin is generally extracted from an animal source, which can result in issues with reproducibility as well as pathogen transmittance. Therefore, we have investigated the potential of a recombinant peptide based on collagen I (RCPhC1) for tissue engineering applications and more specifically for adipose tissue regeneration. In the current paper, RCPhC1 was functionalized with photo-crosslinkable methacrylamide moieties to enable subsequent UV-induced crosslinking in the presence of a photo-initiator. The resulting biomaterial (RCPhC1-MA) was characterized by evaluating the crosslinking behaviour, the mechanical properties, the gel fraction, the swelling properties and the biocompatibility. The obtained results were compared with the data obtained for methacrylamide-modified gelatin (Gel-MA). The results indicated that the properties of RCPhC1-MA networks are comparable to those of animal-derived Gel-MA. RCPhC1-MA is thus an attractive synthetic alternative for animal-derived Gel-MA and is envisioned to be applicable for a wide range of tissue engineering purposes.
1. Introduction
Gelatin, a single-stranded protein extracted from collagen, is widely used for various biomedical applications due to its excellent biological characteristics and tunable physical properties upon chemical functionalization.1–4 To date, animal-derived gelatin has frequently been used to produce cell-interactive scaffolds for the regeneration of adipose tissue.5–7 This type of tissue is often applied to restore volume loss in patients suffering from congenital defects, trauma or surgical deformities.8 However, there are increasing concerns associated with the use of materials derived from animal sources for applications in modern healthcare. The drawbacks include problems with product reproducibility due to batch to batch variations and the risk of pathogen transmittance including prions.9,10 We hypothesize that a recombinant peptide based on human collagen type I could be an attractive alternative to animal-derived gelatin. RCPhC1 is produced by a yeast fermentation process and contains no animal-derived components. The peptide is highly reproducible and has a uniform molecular weight distribution of around 51 kDa. Furthermore, the peptide is enriched with cell-interactive arginine–glycine–aspartic acid (RGD) tripeptide sequences in its backbone, resulting in a good cell interaction.11,12 From literature, it is known that RCPhC1 is suitable for use in in vivo experiments.13Gelatin and RCPhC1 dissolve under physiological conditions, which implies that both materials should be chemically crosslinked to avoid dissolution at body temperature.14,15 In the present work, the amine groups of RCPhC1 and gelatin were modified with photo-crosslinkable methacrylamide functionalities (RCPhC1-MA and Gel-MA, respectively) to avoid the use of potentially toxic crosslinkers such as glutaraldehyde and to reduce the long reaction times that are associated with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride-N-hydroxysuccinimide (EDC-NHS) crosslinking (30 min versus 16 h, respectively).16,17 Furthermore, the applied RCPhC1 contains 1.7 times more amine moieties that can be modified compared to the animal-derived gelatin. Subsequently, the modified materials were crosslinked into 2D hydrogel films upon UV-irradiation in the presence of a suitable photo-initiator. Lithium(2,4,6-trimethylbenzoyl)phenylphosphinate (Li-TPO-L or LAP) was applied in the experiments as a photo-initiator (PI) instead of the more often used PI Irgacure 2959 (I-2959). The latter PI has a molar extinction coefficient of 4 M−1 cm−1 at 365 nm, while Li-TPO-L has a molar extinction coefficient between 200 and 300 M−1 cm−1 at 350–380 nm, which results in a superior reactivity compared to I-2959.18–20 The developed hydrogels were physico-chemically characterized by gel fraction determination, swelling experiments and mechanical tests as well as in vitro biological assays and cell encapsulation experiments.
The aim of the present study was to investigate to what extent RCPhC1-MA effectively exhibits similar or superior characteristics compared to animal-derived Gel-MA. We hypothesized that RCPhC1-MA could be an attractive alternative to animal-derived Gel-MA in terms of reproducibility, processing, mechanical strength, swelling properties and biocompatibility.
2. Experimental section
2.1 Materials
RCPhC1, commercially available as Cellnest™, was kindly provided by Fujifilm Manufacturing Europe B.V. Gelatin type B, isolated from bovine hides, was kindly supplied by Rousselot (Ghent, Belgium). Methacrylic anhydride and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (Diegem, Belgium). Potassium phosphate monobasic (KH2PO4) and sodium phosphate dibasic (Na2HPO4) were obtained from Acros Organics (Geel, Belgium). All 1H-NMR spectra were recorded in deuterium oxide (D2O) purchased from Euriso-top (Saint-Aubin Cedex, France). The photo-initiator lithium(2,4,6-trimethylbenzoyl)phenylphosphinate (Li-TPO-L) was prepared according to the protocol reported by Markovic et al.20,21 Dialysis membranes, Spectra/Por® (molecular weight cut-off (MWCO) 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), were obtained from Polylab (Antwerp, Belgium).
000 g mol−1), were obtained from Polylab (Antwerp, Belgium).
2.2 Derivatization of RCPhC1 and gelatin
The development of methacrylamide-modified RCPhC1 (RCPhC1-MA) and gelatin (Gel-MA) was performed according to the protocol for Gel-MA of Van Den Bulcke et al.14 Briefly, 10 w/v% RCPhC1 (1 g, 0.65 mmol amines) or gelatin (1 g, 0.39 mmol amines) was dissolved in a 0.1 M phosphate buffer (pH 7.8) at 40 °C (Table 1). Subsequently, 1 equivalent or 2.5 equivalents of methacrylic anhydride with respect to the number of amines of RCPhC1 and gelatin, respectively, was added dropwise to the solution followed by reaction for 1 h under continuous mechanical stirring. The excess of unreacted methacrylic anhydride and methacrylic acid formed during the reaction were removed via dialysis (MWCO 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) against distilled water for 24 h at 40 °C. Finally, the materials were isolated by freezing at −20 °C and lyophilized (Christ freeze-dryer alpha I-5).
000 g mol−1) against distilled water for 24 h at 40 °C. Finally, the materials were isolated by freezing at −20 °C and lyophilized (Christ freeze-dryer alpha I-5).
| Amino acid | RCPhC1 (mmol g−1) | Gelatin (mmol g−1) |
|---|---|---|
| Ala | 1.72 | 0.98 |
| Cys | 0.00 | 0.00 |
| Asp | 0.65 | 0.38 |
| Glu | 0.47 | 0.63 |
| Phe | 0.00 | 0.11 |
| Gly | 3.73 | 2.95 |
| His | 0.00 | 0.04 |
| Ile | 0.12 | 0.13 |
| Lys | 0.65 | 0.24 |
| Leu | 0.65 | 0.25 |
| Met | 0.18 | 0.06 |
| Asn | 0.00 | 0.00 |
| Pro | 1.95 | 1.25 |
| Gln | 0.23 | 0.00 |
| Arg | 0.65 | 0.39 |
| Ser | 0.00 | 0.26 |
| Thr | 0.00 | 0.18 |
| Val | 0.18 | 0.22 |
| Trp | 0.00 | 0.00 |
| Tyr | 0.00 | 0.01 |
| Ornithine | 0.00 | 0.07 |
| Hydroxylysine | 0.00 | 0.08 |
2.3 Characterization of the hydrogel building block precursors
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1). Furthermore, the MW of RCPhC1 and RCPhC1-MA was determined using an Ab Sciex 4800+ proteomics matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF/TOF) analyzer (SCIEX, Framingham, MA, USA). The measurements were performed in positive ion linear mode (2000 scans). The material was dissolved in water (1 mg mL−1) and 1 μL of each sample was spotted on the target plate and mixed with 1 μL of matrix α-cyano-4-hydroxycinnamic acid (HCCA) (10 mg mL−1 water/acetonitrile (ACN) (30
300 g mol−1). Furthermore, the MW of RCPhC1 and RCPhC1-MA was determined using an Ab Sciex 4800+ proteomics matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF/TOF) analyzer (SCIEX, Framingham, MA, USA). The measurements were performed in positive ion linear mode (2000 scans). The material was dissolved in water (1 mg mL−1) and 1 μL of each sample was spotted on the target plate and mixed with 1 μL of matrix α-cyano-4-hydroxycinnamic acid (HCCA) (10 mg mL−1 water/acetonitrile (ACN) (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70), 0.1% formic acid).
70), 0.1% formic acid).
2.4 Preparation of hydrogel films via film casting
The hydrogel films were prepared starting from 5 or 10 w/v% RCPhC1-MA or Gel-MA solutions in double distilled water at 40 °C. Next, 2 mol% Li-TPO-L (relative to the amount of methacrylamide present) was added. Subsequently, the solutions were injected between two parallel glass plates covered with a Teflon release sheet and separated by a 1 mm thick silicone spacer. Chemically crosslinked hydrogel films were obtained by irradiating the plates from both sides with UV-A light (365 nm, 5 mW cm−2) for 30 min.2.5 Physico-chemical characterization of the hydrogels
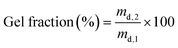 | (1) |
 | (2) |
2.6 In vitro biological evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells in 1 mL of EGM-2 was seeded per well and allowed to attach overnight. The following day, the first microscopy and metabolic activity assays were performed.
000 cells in 1 mL of EGM-2 was seeded per well and allowed to attach overnight. The following day, the first microscopy and metabolic activity assays were performed.
Primary human bone marrow derived cells (MSCs) were isolated and characterized as previously described.25 The cells were cultured at 37 °C in a 5% CO2 atmosphere in low glucose Dulbecco's modified Eagle's medium (Sigma Aldrich, St. Louis, MO) supplemented with 10 v/v% fetal calf serum (Biochrom AG, Berlin, Germany), 1 v/v% penicillin/streptomycin (Biochrom), and 1 v/v% GlutaMAX™ (Thermo Fischer Scientific, Waltham, MA). The cells were passaged at 70–80% confluence and passages 3–4 were used for the experiments. To assess cell morphology, MSCs were seeded onto the hydrogel films (diameter: 8 mm; height: 0.5 mm) at a density of 5 × 104 cells per film using custom built PDMS molds (SYLGARD® 170). After overnight attachment, the hydrogel films were washed twice with PBS and transferred to well plates. At days 1, 3, and 7 after seeding, the cells were fixed with 4% formaldehyde, permeabilized with 0.1% w/v Saponin in 3% BSA-PBS for 10 min and incubated with Alexa Fluor 488® Phalloidin (Thermo Fischer Scientific) according to the manufacturer's instructions. The nuclei were visualized with DAPI. Images of the stained cells were acquired using an inverted fluorescence microscope (Leica). The cell spreading area was quantified using Fiji ImageJ software.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per droplet. Next, the slides were placed in the UV chamber in order to crosslink the gel, as previously described (see Section 2.6.3). The obtained hydrogels were soaked in EGM-2 and placed in the incubator. The samples were incubated for 7 days with microscopy and metabolic activity assays performed regularly.
000 cells per droplet. Next, the slides were placed in the UV chamber in order to crosslink the gel, as previously described (see Section 2.6.3). The obtained hydrogels were soaked in EGM-2 and placed in the incubator. The samples were incubated for 7 days with microscopy and metabolic activity assays performed regularly.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio with medium. In a 12-well plate with different surfaces, 500 μL of the dilution was applied, and on the 3D structures in the μ-slides, 300 μL of the dilution was used per well followed by incubation for 2 h in a cell culture incubator. After incubation, 100 μL of the dilutions was taken out in duplicate and put into a 96-well plate. The leftover PrestoBlue was aspirated and fresh medium was pipetted into the wells with cells. The fluorescence of the supernatants in the 96-well plate was measured using a plate reader (Synergy BioTek, excitation 560 nm, emission 590 nm). The measured fluorescence signals were corrected for background fluorescence. The cells used in this experiment were labelled with a green fluorescent protein to enable the observation of cell viability, proliferation and distribution on and within the material.
10 ratio with medium. In a 12-well plate with different surfaces, 500 μL of the dilution was applied, and on the 3D structures in the μ-slides, 300 μL of the dilution was used per well followed by incubation for 2 h in a cell culture incubator. After incubation, 100 μL of the dilutions was taken out in duplicate and put into a 96-well plate. The leftover PrestoBlue was aspirated and fresh medium was pipetted into the wells with cells. The fluorescence of the supernatants in the 96-well plate was measured using a plate reader (Synergy BioTek, excitation 560 nm, emission 590 nm). The measured fluorescence signals were corrected for background fluorescence. The cells used in this experiment were labelled with a green fluorescent protein to enable the observation of cell viability, proliferation and distribution on and within the material.
3. Results and discussion
3.1 Methacrylation of RCPhC1 and gelatin
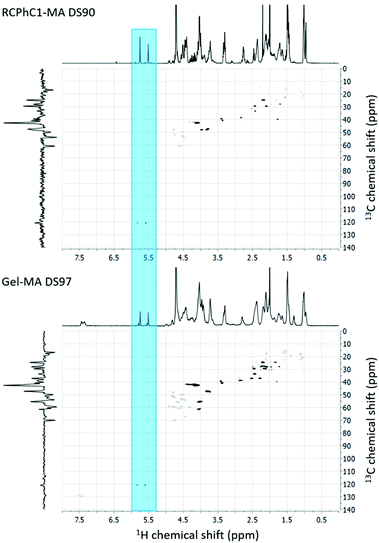
RCPhC1 and gelatin were modified with methacrylic anhydride to introduce photo-crosslinkable functionalities along the backbone enabling subsequent UV-induced crosslinking in the presence of a suitable photo-initiator, Li-TPO-L (Fig. 1). The 1H–13C HSQC NMR the spectra only showed peaks at 5.8 and 5.6 ppm in the 1H NMR spectrum and at 120.9 and 120.8 ppm in the 13C NMR spectrum, which correspond to the vinyl protons of the methacrylamide functionalities. There are no signals at 5.8 and 6.2 ppm and at 130.9 ppm in the 1H and 13C spectra, respectively, which can be assigned to the vinyl protons of methacrylate functionalities, indicating that only the amine moieties are modified and not the hydroxyl groups.26 The degree of substitution (DS) of both materials was quantified via1H-NMR spectroscopy by comparing the integration of the characteristic peaks of the vinyl protons of the methacrylamide functionalities at 5.5–6.0 ppm to the integration of the inert hydrogels of Val, Leu and Ile at 1.0 ppm (Fig. 2). During the modification of RCPhC1-MA, 1 equivalent of methacrylic anhydride was added with respect to the primary amines of the lysine moieties resulting in a DS of 90% (0.60 mmol methacrylamide per g RCPhC1). Furthermore, 2.5 equivalents of methacrylic anhydride were added with respect to the primary amines of lysine, hydroxylysine and ornithine for the modification of Gel-MA, which resulted in a DS of 97% (0.38 mmol methacrylamide per g gelatin). The latter result was in agreement with previous results obtained by Van Hoorick et al.15 Because RCPhC1 has 1.7 times more amine groups per gram, the number of photo-crosslinkable functionalities is also 1.6 times higher compared to Gel-MA.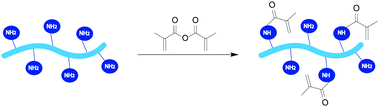 | ||
| Fig. 1 Reaction scheme for the modification of RCPhC1 and gelatin with 1 or 2.5 equivalents of methacrylic anhydride, respectively, in a phosphate buffer (pH 7.8) at 40 °C. | ||
3.2 Assessment of the physical crosslinking behaviour of the hydrogel precursors
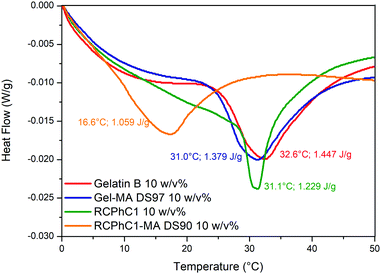
Both gelatin and RCPhC1 exhibit an upper critical solution temperature (UCST), implying that below this temperature, the biopolymers form triple helices. Several factors influence the UCST including the hydrophilicity/hydrophobicity ratio, the molecular weight and the number of incorporated chemical functionalities.14 Visual observations indicated that RCPhC1-MA remained soluble at room temperature. This remarkable observation was further investigated by using differential scanning calorimetry (DSC). Fig. 3 shows that similar melting temperatures (∼30 °C) were recorded for gelatin, Gel-MA and RCPhC1. The melting temperature for RCPhC1-MA is much lower, namely ∼17 °C. Furthermore, the denaturation enthalpy associated with the different materials also differs (Fig. 3). From literature, it is known that the denaturation enthalpy is proportional to the number of hydrogen bonds present, which are responsible for triple helix formation.27 The incorporation of methacrylamide functionalities along the backbone of gelatin and RCPhC1 resulted in a 5% and a 14% decrease in hydrogen bonds, respectively. We hypothesize that the higher amount of double bonds per chain and the lower molecular weight of RCPhC1-MA compared to Gel-MA (53 versus 91 kDa, respectively) have an impact on the reduced triple helix formation of RCPhC1-MA. The latter is due to the fact that a higher amount of photo-crosslinkable moieties and the lower molecular weight resulted in less entanglements between the RCPhC1-MA thereby hampering the triple helix formation. This observation is in accordance with previous results obtained for animal-derived gelatin by Van Hoorick et al., who investigated the physical crosslinking behaviour of different gelatin derivatives including Gel-MA and Gel-MA-AEMA.273.3 Determination of the mechanical properties of the crosslinked hydrogels via rheology
Rheology experiments were performed to determine whether or not the different number of photo-crosslinkable functionalities for RCPhC1-MA and Gel-MA has an influence on the final mechanical properties of the crosslinked hydrogels. In this respect, the storage modulus G′ was monitored as this describes the elastic behavior of a material, which is related to the number of crosslinks present. It is known from literature that G′ becomes higher upon increasing the number of double bonds in the precursors, thereby resulting in a stronger network.28 As anticipated, a clear difference can be observed between the 5 w/v% and 10 w/v% hydrogels (e.g. a G′ of 60 Pa for 5 w/v% and 6000 Pa for 10 w/v% RCPhC1-MA) (Fig. 4). Films formed starting from a higher biopolymer concentration exhibit a higher G′ due to the increased number of photo-crosslinkable moieties that can be chemically crosslinked upon UV irradiation. Furthermore, the results showed that the G′ of RCPhC1-MA and Gel-MA is different for a concentration of 5 w/v% (60 Pa versus 350 Pa respectively), while it is in the same range for 10 w/v% (∼6000 Pa) (Fig. 4). It can be anticipated that the photo-crosslinking of 5 w/v% RCPhC1-MA is less efficient due to its lower molecular weight (53 kDa for RCPhC1-MA versus 91 kDa for Gel-MA), resulting in less entanglements between the RCPhC1-MA chains. At higher polymer concentrations, the higher number of photo-crosslinkable functionalities of RCPhC1-MA can compensate for the lower amount of entanglements resulting in a comparable G′ for both materials after crosslinking. This observation is in agreement with earlier reports described in the literature. Indeed, Gao et al. showed that materials with a lower molecular weight have fewer entanglements, resulting in lower mechanical strength.29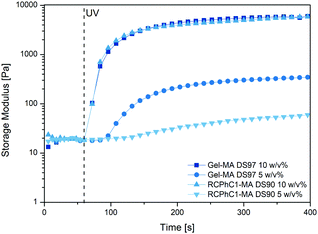 | ||
| Fig. 4 Evolution of the storage modulus G′ of RCPhC1-MA DS90 (5 and 10 w/v%) and Gel-MA DS97 (5 and 10 w/v%) as a function of time upon application of UV light at 37 °C. | ||
3.4 Gel fraction and swelling experiments
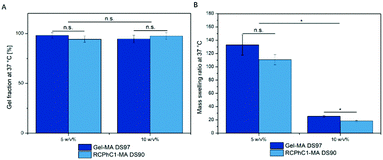
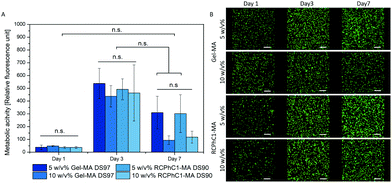
Similar gel fractions exceeding 90% were obtained for both RCPhC1-MA- and Gel-MA-based hydrogels indicating successful hydrogel film formation (Fig. 5). No significant differences (p < 0.05) were observed for any of the developed hydrogel films. The results showed that for the 5 w/v% as well as the 10 w/v% concentration, stable hydrogels were formed. The obtained results are in accordance with previous data reported by Van Nieuwenhove et al. and Van Hoorick et al.27,30 The gel fraction results provided a first indication that the UV crosslinking was efficient. HR-MAS 1H-NMR spectroscopy experiments were performed to further substantiate the latter observation. This technique was used to gain insight into the double bond conversion by quantifying the number of double bonds that were consumed during UV-induced crosslinking according to the protocol of Van Vlierberghe et al.31 A double bond conversion of around 95% for RCPhC1-MA was determined, while it was around 70% for Gel-MA. The latter result was in agreement with the double bond conversion of 63% for Gel-MA-based hydrogels reported earlier by Van Nieuwenhove et al.30 We hypothesize that the lower molecular weight of RCPhC1-MA contributes to a higher mobility of the photo-initiator and the biopolymer resulting in a higher double bond conversion.Hydrogels are ideal candidates for tissue engineering applications because they can closely mimic the aqueous environment of the extracellular matrix (ECM). Therefore, the mass swelling ratio is one of the important characteristics to determine whether or not a material is suitable to function as an ECM mimic.32 The results of the swelling experiments indicated that both hydrogels can absorb large amounts of water. The mass swelling ratio q decreases from 111 to 18 for RCPhC1-MA and from 133 to 26 for Gel-MA depending on the biopolymer concentration (Fig. 5). As anticipated, the water uptake capacity of the hydrogel film decreases with increasing concentration because of the formation of a more densely crosslinked network due to the higher number of photo-crosslinkable functionalities present. Interestingly, 10 w/v% RCPhC1-MA swells significantly (p < 0.05) less compared to the same concentration of Gel-MA. We hypothesize that the latter is due to the fact that RCPhC1-MA has 24% less hydrophilic moieties such as aspartic acid and glutamic acid that attract water and contains 20% more hydrophobic functionalities (i.e. Ile, Leu, Val, Met and Phe) compared to Gel-MA (Table 1). The latter observation is in accordance with previous literature reports. Vázquez et al. reported that the water uptake capacity of a material decreases with increasing amounts of hydrophobic moieties present.33 In addition, from the literature, it is also known that the isoelectric point (IEP) can have an influence on the swelling behaviour of hydrogels.34 Gelatin B has an IEP of 5, while RCPhC1 has an IEP of 10.32
3.5 In vitro biological evaluation
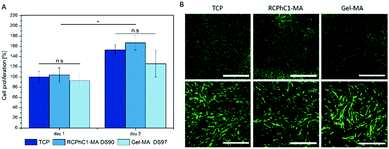
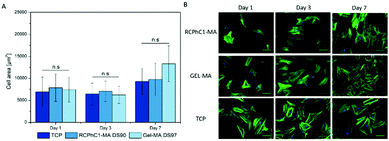
The present study also focused on assessing the material versatility towards different stem cell types. Both adipose tissue and bone marrow derived stem cells have the ability to differentiate into the adipogenic lineage.39 Therefore, additional cell seeding experiments with bone marrow derived MSCs, seeded onto RCPhC1-MA and Gel-MA hydrogel films, were performed. The results indicated that the MSCs also nicely attached to RCPhC1-MA and Gel-MA due to the presence of the cell-interactive RGD tripeptides within the amino acid sequence. After day 1, the MSCs also started to proliferate and exhibited their spindle-like morphology (Fig. 7). The latter observation is in accordance with previous literature reports about culturing of MSCs on Gel-MA.40
4. Conclusions
The present manuscript reported on the physical crosslinking behaviour of the hydrogel precursors as well as the mechanical properties, the gel fraction and the swelling properties of RCPhC-MA and Gel-MA. Furthermore, in vitro biological assays and cell encapsulation experiments were performed. The results showed that the physical crosslinking behaviour of the modified RCPhC1 is affected by the presence of the photo-crosslinkable functionalities that affect the triple helix formation. The gel fraction together with the crosslinking efficiency results indicated that for both materials, the UV crosslinking was successful and that stable hydrogel films were formed. In addition, the mechanical properties of the final hydrogels are in the same range at a polymer concentration of 10 w/v%. However, the water uptake capacity of crosslinked RCPhC1-MA is lower than that for Gel-MA due to its lower hydrophilicity. In vitro biological evaluation indicated that RCPhC1-MA is biocompatible towards ASCs and MSCs. In addition, cell encapsulation experiments showed that the encapsulated ASCs survived the encapsulation process and started to proliferate within the hydrogel, which is an indication that RCPhC1-MA is an excellent ECM mimic with a comparable stem cell response to that of Gel-MA. It can be concluded that the properties of RCPhC1-MA are comparable with those of the gold standard Gel-MA after crosslinking. RCPhC1-MA is thus an attractive synthetic alternative to animal-derived Gel-MA for different biomedical applications including adipose tissue engineering.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Mr Tim Courtin for his assistance in recording the 1H-NMR spectra. The work of L. Tytgat was supported by the Research Foundation Flanders (FWO) under a PhD research fellowship (1S26616N). S. Van Vlierberghe would like to acknowledge FWO (Belgium) for financial support in the form of research grants (G005616N, G0F0516N, FWOKN273 and G044516N). A. Ovsianikov would like to acknowledge the financial support from the Austrian Science Funds (FWF Project No. I2444-N28). F. Bray and C. Rolando would like to acknowledge the IBiSA network for financial support of the USR 3290 (MSAP) proteomics facility.References
- T. Billiet, E. Gevaert, T. De Schryver, M. Cornelissen and P. Dubruel, Biomaterials, 2014, 35, 49–62 CrossRef CAS PubMed.
- J. Lewandowska-Łańcucka, K. Mystek, A. Mignon, S. Van Vlierberghe, A. Łatkiewicz and M. Nowakowska, Carbohydr. Polym., 2017, 157, 1714–1722 CrossRef PubMed.
- L. J. Luo, J. Y. Lai, S. F. Chou, Y. J. Hsueh and D. H. K. Ma, Acta Biomater., 2017, 65, 123–136 CrossRef PubMed.
- K. Yue, G. T. Santiago, A. Tamayol, N. Annabi, A. Khademhosseini, W. Hospital and S. Arabia, Biomaterials, 2016, 73, 254–271 CrossRef PubMed.
- M. K. Phull, T. Eydmann, J. Roxburgh, J. R. Sharpe, D. J. Lawrence-Watt, G. Phillips and Y. Martin, J. Mater. Sci.: Mater. Med., 2013, 24, 461–467 CrossRef CAS PubMed.
- K. H. Chang, H. T. Liao and J. P. Chen, Acta Biomater., 2013, 9, 9012–9026 CrossRef CAS PubMed.
- B. W. Booth, C.-C. Yang and K. J. L. Burg, J. Biomater. Sci., Polym. Ed., 2012, 37–41 Search PubMed.
- C. J. Poon, M. V. Pereira, E. Cotta, S. Sinha, J. A. Palmer, A. A. Woods, W. A. Morrison and K. M. Abberton, Acta Biomater., 2013, 9, 5609–5620 CrossRef CAS PubMed.
- K. M. Pawelec and S. G. J. M. Kluijtmans, ACS Biomater. Sci. Eng., 2017, 3, 1100–1108 CrossRef CAS.
- D. Olsen, C. Yang, M. Bodo, R. Chang, S. Leigh, J. Baez, D. Carmichael, M. Perälä, E. R. Hämäläinen, M. Jarvinen and J. Polarek, Adv. Drug Delivery Rev., 2003, 55, 1547–1567 CrossRef CAS PubMed.
- A. J. García, Biomaterials, 2005, 26, 7525–7529 CrossRef PubMed.
- M. Parvizi, J. A. Plantinga, C. A. F. M. Van Speuwel-Goossens, E. M. W. M. Van Dongen, S. G. J. M. Kluijtmans and M. C. Harmsen, J. Biomed. Mater. Res., Part A, 2016, 104, 503–516 CrossRef CAS PubMed.
- A. Tuin, S. G. Kluijtmans, J. B. Bouwstra, M. C. Harmsen and M. J. A. Van Luyn, Tissue Eng., Part A, 2010, 16, 1811–1821 CrossRef CAS PubMed.
- A. I. Van Den Bulcke, B. Bogdanov, N. De Rooze, E. H. Schacht, M. Cornelissen and H. Berghmans, Biomacromolecules, 2000, 1, 31–38 CrossRef CAS.
- J. Van Hoorick, H. Declercq, A. De Muynck, A. Houben, L. Van Hoorebeke, R. Cornelissen, J. Van Erps, H. Thienpont, P. Dubruel and S. Van Vlierberghe, J. Mater. Sci.: Mater. Med., 2015, 26, 247 CrossRef PubMed.
- H.-W. Sung, R.-N. Huang, L. L. H. Huang and C.-C. Tsai, J. Biomater. Sci., Polym. Ed., 1999, 10, 63–78 CrossRef CAS PubMed.
- A. Sharma, S. Bhat, T. Vishnoi, V. Nayak and A. Kumar, BioMed Res. Int., 2013, 2013, 1–15 Search PubMed.
- S. Benedikt, J. Wang, M. Markovic, N. Moszner, K. Dietliker, A. Ovsianikov, H. Grützmacher and R. Liska, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 473–479 CrossRef CAS.
- B. D. Fairbanks, M. P. Schwartz, C. N. Bowman and K. S. Anseth, Biomaterials, 2009, 30, 6702–6707 CrossRef CAS PubMed.
- M. Markovic, J. Van Hoorick, K. Hölzl, M. Tromayer, P. Gruber, S. Nürnberger, P. Dubruel, S. Van Vlierberghe, R. Liska and A. Ovsianikov, J. Nanotechnol. Eng. Med., 2015, 6, 0210011 Search PubMed.
- M. Noe, R. Henne and A. Maase, Ger. Pat. Appl., WO2003068784A2, 2003.
- J. R. Prado and S. Vyazovkin, Macromol. Chem. Phys., 2014, 215, 867–872 CrossRef CAS.
- L. Knezevic, M. Schaupper, S. Mühleder, K. Schimek, T. Hasenberg, U. Marx, E. Priglinger, H. Redl and W. Holnthoner, Front. Bioeng. Biotechnol., 2017, 5, 1–12 Search PubMed.
- D. Mandt, P. Gruber, M. Markovic, M. Tromayer, M. Rothbauer, S. Rudi, A. Kratz, S. F. Ali, J. Van Hoorick, W. Holnthoner, S. Mühleder, P. Dubruel, S. Van Vlierberghe, P. Ertl, R. Liska and A. Ovsianikov, Int. J. Bioprint., 2018, 4, 1–12 Search PubMed.
- G. Kasper, J. D. Glaeser, S. Geissler, A. Ode, J. Tuischer, G. Matziolis, C. Perka and G. N. Duda, Stem Cells, 2007, 25, 1985–1994 CrossRef CAS PubMed.
- C. Claaßen, M. H. Claaßen, V. Truffault, L. Sewald, G. E. M. Tovar, K. Borchers and A. Southan, Biomacromolecules, 2018, 19, 42–52 CrossRef PubMed.
- J. Van Hoorick, P. Gruber, M. Markovic, M. Tromayer, J. Van Erps, H. Thienpont, R. Liska, A. Ovsianikov, P. Dubruel and S. Van Vlierberghe, Biomacromolecules, 2017, 18, 3260–3272 CrossRef CAS PubMed.
- G.-J. Graulus, A. Mignon, S. Van Vlierberghe, H. Declercq, K. Fehér, M. Cornelissen, J. C. Martins and P. Dubruel, Eur. Polym. J., 2015, 72, 494–506 CrossRef CAS.
- Y. Gao, L. Duan, S. Guan, G. Gao, Y. Cheng, X. Ren and Y. Wang, RSC Adv., 2017, 7, 44673–44679 RSC.
- I. Van Nieuwenhove, A. Salamon, K. Peters, G. J. Graulus, J. C. Martins, D. Frankel, K. Kersemans, F. De Vos, S. Van Vlierberghe and P. Dubruel, Carbohydr. Polym., 2016, 152, 129–139 CrossRef CAS PubMed.
- S. Van Vlierberghe, B. Fritzinger, J. C. Martins and P. Dubruel, Appl. Spectrosc., 2010, 64, 1176–1180 CrossRef CAS PubMed.
- S. Van Van Vlierberghe, E. Schacht and P. Dubruel, Eur. Polym. J., 2011, 47, 1039–1047 CrossRef.
- B. Vázquez, J. San Roman, C. Peniche and M. E. Cohen, Macromolecules, 1997, 30, 8440–8446 CrossRef.
- C. Qiao, X. Cao and F. Wang, Polym. Polym. Compos., 2012, 20, 53–58 CAS.
- K. Yue, X. Li, K. Schrobback, A. Sheikhi, N. Annabi, J. Leijten, W. Zhang, Y. S. Zhang, D. W. Hutmacher, T. J. Klein and A. Khademhosseini, Biomaterials, 2017, 139, 163–171 CrossRef CAS PubMed.
- G. Eke, N. Mangir, N. Hasirci, S. MacNeil and V. Hasirci, Biomaterials, 2017, 129, 188–198 CrossRef CAS PubMed.
- J. W. Nichol, S. T. Koshy, H. Bae, C. M. Hwang, S. Yamanlar and A. Khademhosseini, Biomaterials, 2010, 31, 5536–5544 CrossRef CAS PubMed.
- K. Yue, G. Trujillo-De Santiago, M. Mois Es Alvarez, A. Tamayol, N. Annabi and A. Khademhosseini, Biomaterials, 2015, 73, 254–271 CrossRef CAS PubMed.
- I. Van Nieuwenhove, A. Salamon, S. Adam, P. Dubruel, S. Van Vlierberghe and K. Peters, Carbohydr. Polym., 2017, 161, 295–305 CrossRef CAS PubMed.
- R. Levato, W. R. Webb, I. A. Otto, A. Mensinga, Y. Zhang, M. van Rijen, R. van Weeren, I. M. Khan and J. Malda, Acta Biomater., 2017, 61, 41–53 CrossRef CAS PubMed.
- Y. Gu, L. Zhang, X. Du, Z. Fan, L. Wang, W. Sun, Y. Cheng, Y. Zhu and C. Chen, J. Biomater. Appl., 2018, 33, 609–618 CrossRef CAS PubMed.
- W. Liu, M. A. Heinrich, Y. Zhou, A. Akpek, N. Hu, X. Liu, X. Guan, Z. Zhong, X. Jin, A. Khademhosseini and Y. S. Zhang, Adv. Healthcare Mater., 2017, 6, 1–11 Search PubMed.
Footnote |
| † These authors are associated with the Austrian Cluster for Tissue Regeneration (http://www.tissue-regeneration.at). |
| This journal is © The Royal Society of Chemistry 2019 |