Open Access Article
Open Access ArticleBibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications†
Mirta
Jiménez
a,
Cristina
Abradelo
a,
Julio
San Román
bc and
Luis
Rojo
 *bc
*bc
aUnversidad CEU San Pablo, Spain
bInstituto de Ciencia y tecnología de Polímeros, CSIC, Spain. E-mail: rojodelolmo@ictp.csic.es
cConsorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina Spain, Spain
First published on 12th February 2019
Abstract
Musculoskeletal disorders such as osteoporosis and rheumatoid arthritis are responsible for more than 25k deaths every year in the European Union and constitute a chronic burden on the individuals who suffer from this condition. There is no medical cure for these diseases and there are many therapies applied which have limited effectiveness and severe side effects over time. Regenerative therapies are being studied as a potential treatment for musculoskeletal diseases and are known for their upgrading effects of the natural healing processes carried out in the human body. It is believed that both strontium and zinc play an essential role in bone and cartilage tissue formation, which has led many scientists to study the effect of including these elements to promote tissue formation and inhibit its resorption. In this review, a deep analysis is undertaken of the most relevant developments in strontium and zinc based regenerative therapies that have occurred in the last five years, taking into consideration only those studies reporting significant progress towards real clinical applications. This review brings up to date the state of the art of strontium and zinc based regenerative therapies as it is believed that both have a promoting effect on tissue formation and have an essential role inhibiting resorption in musculoskeletal disorders.
1. Introduction
Bone and cartilage health and repair consist of maintaining the balance between tissue resorption and formation. Various musculoskeletal diseases such as Paget's disease, osteoarthritis, rheumatoid arthritis, osteogenesis imperfecta and osteoporosis derive from an imbalance of these processes.1,2 This group of diseases constitutes a major burden on individuals and health systems being responsible for more than 25k deaths every year in the European Union,3 where 66% were women.4 This situation encourages European research priorities to improve advanced therapies able to regenerate and restore tissues, organs and functions.5 Tissue loss, particularly of bone and cartilage, continues to be a challenge;6 recent statistics reveal that one in three women and one in five men will have an osteoporotic fracture over the age of fifty.7 When bone tissue loss is present, fracture treatment carries important challenges due to the high risk of mortality in the first year.8The most common form of musculoskeletal disorders is osteoporosis, also known as “primary osteoporosis”, which is the result of bone loss due to bone resorption, which consists mainly of the differentiation of a stromal cell/osteoblast or a macrophage into an osteoclast,9 and deterioration of bone structure as people age.10 It is believed that osteoclastic bone resorption is regulated by the RANKL/osteoprotegerin (OPG)/RANK pathway, while osteoblastic bone formation is controlled by the canonical Wnt/β-catenin signalling pathway.11–13 There are multiple risk factors for osteoporosis including sex, obesity, genetic predisposition, inflammation, stress and lifestyle.14 The current therapies used for osteoporosis include: vitamin D and calcium supplements, bisphosphonates, teriparatide (TPH), hormone replacement therapy, selective oestrogen receptor modulators (SERMs), RANK ligand inhibitors and strontium ranelate, in which the safety issues and side effects are quite concerning.15
Regenerative therapies imply the replacement or regeneration of human cells, tissues or organs with the purpose of returning the patient to full health,16 by using concrete factors to upgrade the natural healing potential.17 Currently there are still many diseases with no effective medical therapy which could be treated with tissue replacement.18 Several strategies can be used: cell-based therapy, implantation of scaffolds seeded with cells and biomaterials which can be enriched with bioactive factors.19
Strontium (Sr) is an alkaline earth element with close similarities with calcium (Ca) due to its bone-seeking properties. It has been found that vitamin D induces Sr absorption while Ca inhibits it.20 Sr plays important physicochemical and biological roles in the application of bone repair materials;21 it is known to be involved in the human bone mineral metabolism by inducing the expression of osteogenic-related genes,22 differentiation markers, and proliferation and reducing apoptosis levels.23 Moreover, Pasqualetti S. et al. have demonstrated that Sr also affects bone mineralization during skeletal development in zebrafish embryos.24 For instance, strontium ranelate (SR) is currently used as a treatment against bone fractures in post-menopausal women.25 Although slight toxicity in some of the patients was reported,26 the latest studies report a low dose of Sr promotes osteogenesis, but high doses can, in explicit cases, induce apoptosis.27 Additionally, Sr enriched biomaterials have been shown to induce osteogenesis and new bone formation (Fig. 1).28
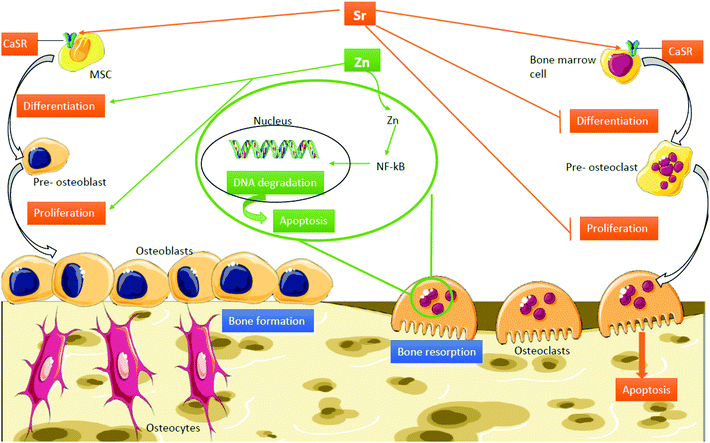 | ||
| Fig. 1 Mechanisms of action of Sr and Zn in bone. Abbreviations: CaSR, calcium sensing receptor; MSC, mesenchymal stem cell; NF-kb, nuclear factor kappa-light-chain-enhancer of activated B cells. | ||
On the other hand, it is established that zinc (Zn) is an essential cofactor for enzymes involved in DNA, RNA and protein synthesis such as bone matrix components and is an essential component of the cell membrane repair machinery and musculoskeletal matrix regeneration.29 The role of Zn in bone is twofold: firstly, Zn plays a structural role in the bone matrix and on the other hand, it is involved in the preservation of bone mass by stimulating bone formation by osteoblasts and inhibition of bone resorption by osteoclasts (Fig. 1).30 Furthermore, it has been confirmed that Zn deficiency leads to growth and gonadal development retardation, either for a deficient Zn intake or a defect in absorption.31,32 Not only does Zn act as an inhibitor of bone tissue loss but also plays an important role in cartilage II and SOX9 gene expression, cartilage matrix metabolism and inflammatory-related diseases carried out by Zn-dependent matrix metalloproteinases (MMPs).33–36
Since these two cations are easily available from accessible resources and they do not suffer degradation reactions during processing, storage and transport, there is an important research activity developing promising alternatives to more unstable, and sometimes prohibitive, growth factor and signalling biomacromolecule based therapies for real clinical applications. Thus, the aims of this review are to provide an expanded analysis of the scientific literature focused on the application of Sr and Zn as bioactive agents in musculoskeletal regenerative therapies in the last five years, and to deliver a valuable guide for a wide range of researchers in the fields of medicine, chemistry and material and pharmaceutical sciences interested in the biological role and significance of Sr and Zn containing materials in regenerative medicine.
2. Tissue engineering based on Zn and Sr containing scaffolds
Scaffolds have multiple applications and play an essential role in tissue engineering and can be obtained from a wide variety of fabrication procedures such as 3D printing, electrospinning, cryo-photo-redox-mediated polymerization or sintering. These scaffolds can be made from natural existing polymers, synthetic materials or combinations of both, in order to mimic the physicochemical and biological properties of the damaged or missing tissue by creation of an environment that promotes the capacity of cells to integrate, proliferate and differentiate.37–39A bioactive glass is a biocompatible material that can mimic human bone and induce re-growth. It also can be mixed with other materials such as Sr to promote its bioactivity.40 Nine authors were found to be operating with this biomaterial, though applying different combinations and performing experiments on different cell lines and/or animals: a Sr substituted bioactive glass, where calcium oxide (CaO) was substituted by Sr in three different molar proportions, being 0%, 50% and 100%, using bone marrow stromal cells (BMSCs);41 a sol–gel synthesized partially substituted CaO by SrO (on a 0–10% molar basis) bioactive glass on clonal osteoblast-like mouse calvarial cells (MC3T3-E1);42 a Sr and lithium (Li) co-doped bioactive glass scaffold using all inorganic components (SrCO3 and Li2CO3) on mouse embryonic fibroblast cells (NIH3T3) and further along expanding the experiment to New Zealand white rabbits;43 a Sr containing calcium sulphate (CS)/poly (amino acid) bioactive graft, where Sr and CS were directly added into the scaffold mix performed on MC3T3-E1 cells and as well as on New Zealand white rabbits;44 a porous Sr incorporating mesopore-bioglass (Sr-MBG) scaffold, in comparison to a non-doped mesopore bioglass (MBG) where 15 female Wistar rats were randomly assigned into three groups: (1) control unfilled periodontal defects, (2) MBG alone and (3) Sr-MBG scaffolds;45 Sr doped bioactive phosphate glasses of general composition 40(P2O5)–25(CaO)–5(Na2O)–(30−x)MgO–x(SrO) with x = 0, 1, 5, and 10 where Sr was substituted in magnesium's (Mg) place were analysed using human mesenchymal stem cells (hMSCs);46 Sr and Ca releasing titanium-stabilised phosphate-based glasses of general formula 25(P2O5)–5(Na2O)–5(TiO2)–(35−x) CaO–x(SrO) where x = 0, 3.5, 17.5, and 35 and Sr was substituting Ca in the human osteosarcoma cell line (MG-63);47 Human Umbilical Vein Endothelial Cells (HUVECs) were treated with a phosphate based but also multicomponent bioactive glass: SiO2–P2O5–CaO–SrO–Na2O–MgO–ZnO–K2O, where four groups were assembled, Ca-glass, Sr-glass, Ca–cobalt (Co)-glass and Sr–Co-glass;48 and lastly, phosphate based glasses that contain Sr, Zn and Ca of general formula (P2O5)–(Na2O)–(TiO2)–(CaO)–(SrO) or (ZnO) on MG-63 cells.49 The last four fabricated the bioactive glasses via the melt-quenching technique, which consists of a quenching consecutive to the fusion of two or more oxide components.50
It is well known that the bone extracellular matrix (ECM) is composed of inorganic components such as hydroxyapatite crystals and organic components such as collagen,51 which arrange into nanofibers controlling the behaviour of the cells. Hence, using nanofibers as a biomaterial is a wise decision as it mimics the situation in vivo.52 Weng L. et al. chose to fuse the utility of bioactive glasses and nanofibers, using glass material to fabricate nanofibers substituting Ca for Sr and copper (Cu), creating several scaffolds: undoped or Ca bioactive glass fibres; 50% Sr doped bioactive glass fibers; 1% Cu and 50% Sr doped bioactive glass fibers; and 0.5% Cu and 50% Sr doped bioactive glass nanofibers in adipose tissue-derived mesenchymal stem cells (ADSCs).53 Other Sr doped nanofibers were analysed: poly(ε-caprolactone) (PCL) based nanofibers.54,55 In the first case,54 hMSCs were treated with SrCrO3-electrospun nanofibers where several samples were made: PCL (SrCO3 0%), PCL/SrC10 (SrCO3 10%) and PCL/SrC20 (SrCO3 20%). On the other hand,55 strontium phosphate [Sr3(PO4)2] was added by immersing the nanofibers in a strontium phosphate solution, obtaining three samples: Sr coated PCL nanofibers, PCL + 0.8% Sr nanofibers and PCL-only nanofibers as the control, and they were used to treat stem cells from human exfoliated deciduous teeth (SHEDs).
As already stated, the bone ECM is composed of inorganic components such as hydroxyapatite crystals, so creating a biomaterial using hydroxyapatite and implanting it into an animal gives the advantage of not having any kind of adverse reactions in addition to perfect fitting. Two combinations of hydroxyapatite materials were found: Sr containing nanostructured hydroxyapatite microspheres (SrHA) compared to stoichiometric hydroxyapatite (HA) microspheres were implanted into a sheep,56 and Sr substituted hydroxyapatite (Sr-HA) was obtained by adding Sr2+ ions into a Ca2+ solution; the appropriate amounts of Sr(NO3)2 and Ca(NO3)2·4H2O were dissolved in order to obtain 8% or 50% (molar ratio) of Ca2+ substituted by Sr2+ (8Sr-HA and 50Sr-HA) and further along implanted into mice.57
Bone cements are usually composed of two systems: powder (homopolymer PMMA (polymethyl methacrylate) + BPO (benzoyl peroxide)/ZrO2 (zirconia)/BaSO4 (barium sulphate)); and liquid (monomer MMA + hydroquinone + N,N-dimethyl para-toluidine (DMPT)).58 Cui X. et al.59 offered an innovative proposal by fusing these two concepts: bone cements and bioactive glasses, integrating a Sr containing borate bioactive glass (SrBG) into a bone cement in several concentrations: 10SrBG, 20SrBG and 30SrBG being 0.2, 0.4 and 0.6 g of SrBG respectively and comparing the results with a non-doped PMMA cement. This experiment was carried out on the MC3T3-E1 cell line as well as in vivo, in healthy and osteoporotic Sprague-Dawley rats. On the other hand, a Sr loaded bone cement (Sr-BC) was prepared in different concentrations Sr/(Ca + Sr) = 0%, 2%, 5% in a different study where the experiment was only carried out in vitro on the mouse monocyte cell line (RAW 264.7) and the MC3T3-E1 cell line.60 Further, a cement precursor was found composed of 58 wt% α-tricalcium phosphate, 24 wt% calcium hydrogen phosphate, 8.5 wt% hydroxyapatite and 8.5 wt% strontium carbonate and later mixed with a 4% aqueous disodium hydrogen phosphate solution to assist new bone formation in Sprague-Dawley rats,61 and silicon (Si) and Zn doped brushite cements (BrCs) alone and in combination with insulin like growth factor 1 (IGF-1), coming to four different scaffolds: (IGF-1) BrC, (IGF-1) Si-BrC, (IGF-1) Zn-BrC, and (IGF-1) Si/Zn-BrC cements, on New Zealand white rabbits.62
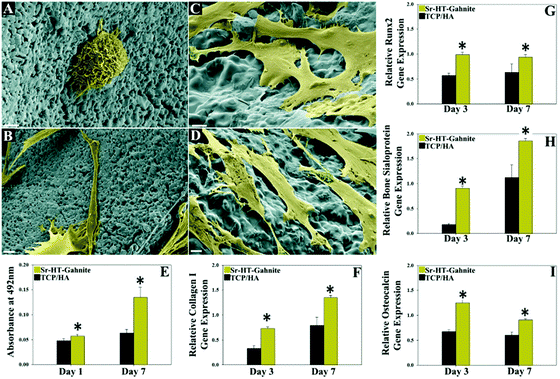
Moreover, scaffolds from very different compositions were found: SrO doped biosilicate scaffolds, fabricated by mixing Mg2SiO4 and CaSiO3 and adding SrO in different ratios, being 0SrO (0 wt%), 0.5SrO (0.5 wt%), 1SrO (1 wt%), 2SrO (2 wt%), and 3SrO (3 wt%), were used to treat MG-63 cells;63 a zinc silicate mineral coated PLLA scaffold compared to a non-coated scaffold and tissue culture plastic (TCPS), cultured with adipocyte derived stem cells (ADSCs);64 a strontium chloride (SrCl2) coated porcine femur cancellous bone derived scaffold (CPB) subsequently coated with polycaprolactone (PCL) obtaining CPB/Sr/PCL on hMSCs;65 a sol–gel method synthesized hybrid scaffold incorporating: phosphate ions, calcium from calcium dichloride (CaCl2·2H2O) and Sr from strontium dichloride hexahydrate (SrCl2·6H2O) was incorporated into human osteoblast cell line (HOB) cultures;66 a Sr folate (SrFO) loaded bio-hybrid porous scaffold obtained by interpenetrating beta tricalcium phosphate (βTCP) and polyethylene glycol dimethacrylate networks in contrast with a βTCP scaffold, which was used in an experiment in human dental pulp stem cells (HDPSCs) as well as in vivo in Wistar rats;67 Wharton's jelly-derived mesenchymal stem cells (WJCs) were treated with either a rod-like nano hydroxyapatite (RN-HA) or a flake-like micro hydroxyapatite (FM-HA) as a coating for a Mg–Zn–Ca alloy scaffold in another study;68 a Collagen type-I (Col-I) coated magnesium–zirconia (Mg–Zr) alloy, containing different quantities of Sr, where the scaffolds were divided into 3 samples: No-Sr, Low-Sr (1.82 wt%) and High-Sr (4.8 wt%) and later implanted into New Zealand white rabbits;69 another Sr containing HA/polylactide composite group with four scaffolds was obtained: CT (control, Sr0/polylactide), SrL (Sr0.5/polylactide), SrM (Sr5/polylactide) and SrH (Sr50/polylactide), which were as well implanted into New Zealand white rabbits;70 and lastly, three different studies chose to use a calcium silicate based bio-ceramic that contains Sr and Zn ions: strontium–hardystonite–gahnite (Sr–HT–gahnite) scaffolds,71–73 since it has been recently studied due to its biocompatibility and exclusive microstructure (Fig. 2). The structure consists of crystalline strontium–hardystonite grains embedded with submicron gahnite crystals (prevents cracks).74 The studies respectively carried out the experiments on human peripheral blood monocytes (hPBM),71 adult stem cells (ASCs) and HUVEC cell lines72 and, lastly, primary human osteoblasts (HOBs) and New Zealand white rabbits.73
3. Osteoinductive titanium surfaces containing Sr
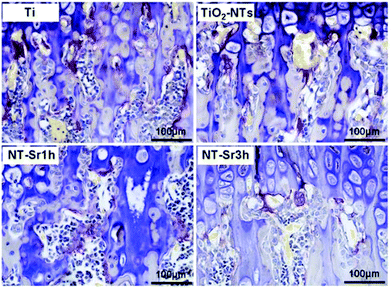
Titanium (Ti) and its alloys have been used in a wide range of clinical applications such as orthopaedic and dental implants. Biocompatibility is the most significant property to be accomplished by a biomaterial to be used in any clinical application. We know that Ti is a chemically and mechanically stable, non-toxic, bone integrating material,75 which however does not fulfil rapid osseointegration regenerative clinical use demands.76 Different surface modifications have been recently described for inclusion of osteogenic elements.77 On this subject, eight articles discussed the possibilities of doping titanium surfaces with Sr, well known for its possibilities to intensify bone regenerating features, such as SrTiO3/TiO276 and Sr loaded titania nanotubes,78 consisting of adding the Sr through a hydrothermal treatment.79,80 In both cases, the RAW 264.7 cell line was used for the experiment in vitro, although B. Mi et al. expanded their experiment in vivo, performing it on Sprague-Dawley rats (Fig. 3).78Furthermore, the immune response to implanted biomaterials is imperative to apprehend whether a material is suitable or not. Knowing that Sr and Ca can induce osteogenesis and abolish inflammation, two papers were found which take advantage of these promising properties: BMSCs and RAW 264.7 cell lines were used in an experiment consisting of Sr and Ca co-doped titanium oxides, where numerous concentration ratios were used to go through the experiment: Ti, Ti-Ca10, Ti-Sr10, Ti-Ca10Sr10, Ti-Ca10Sr5 and Ti-Ca10Sr2.5, obtained by an electrochemical treatment of calcium chloride and strontium dichloride solutions on titanium plates79 and Sr doped calcium (Ca)- and phosphate (P)-containing titanium oxide layers. The Ti oxide layers were modified by the micro-arc oxidation method (MAO), which is known for augmenting biocompatibility and osseointegration in vivo;81 these Sr doped calcium (Ca)- and phosphate(P)-containing titanium oxide layers were also used further along for an experiment in vivo on C57BL/6 mice. In another study, electrolytes with different proportions of Sr: Sr-0M, Sr-0.05M, Sr-0.1M and Sr-0.15M were analysed using MC3T3E1 cells.80 Calcium phosphate (CPS) coated titanium sheets, adding Sr2+ by vertical immersion to reach varying concentrations of Sr2+ in CPS (0, 10, and 1000 μM), were tested on hMSCs looking for osteogenic differentiation;82 a SrO layer on a sandblasted large grit double acid etched (SLA) surface produced by hydrothermal treatment using a Sr containing solution was implanted into New Zealand white rabbits;83 and lastly, titanium implants were induced to release Sr (Sr–Ti) or Mg (Mg–Ti) ions in Japanese white rabbits.84
Sodium alginate (SA) is a promising and efficient biomaterial for bone formation and healing,85,86 and its utility in bone tissue regeneration has been studied as a coating with Sr and dopamine (DOPA) on a titanium (Ti) surface, which are well-known for their potential characteristics for bone tissue engineering; five samples were made: Ti, Ti/DOPA, Ti/DOPA/SA, Ti/DOPA/SA/Sr1 (1 wt% Sr) and Ti/DOPA/SA/Sr5 (5 wt% Sr) and tested using MG-63 cells.87
4. Strontium ranelate based therapies
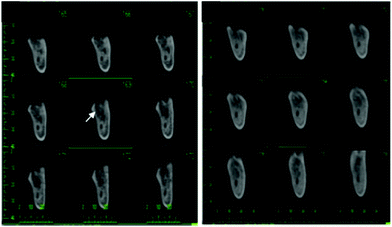
Strontium ranelate (SR) is composed of two atoms of stable Sr combined with organic ranelic acid. It seems to have unique effects of inhibiting bone resorption as well as stimulating bone formation and it currently used as a treatment for osteoporosis and other bone diseases.88 Since it has outstanding properties for bone tissue engineering, various authors decided to evaluate the effects of this compound (Fig. 4): a SR therapy was investigated to assess its effects on fracture frequency and bone mass and strength in mice;89 a SR treatment was examined in a postmenopausal woman with osteonecrosis of the jaw after long-term oral bisphosphonate administration;90 SR in the repair of standardized intrabuccal bone defects in Lewis rats was evaluated;91 the effects of insulin and SR on guided bone regeneration in diabetic Wistar rats were analysed;92 how a previous biophosphonate treatment can modify the effects of SR on bone mineral density was investigated in humans;93 the properties of SR for the stimulation of trabecular bone formation in a Sprague-Dawley rat tibial bone defect healing process;94 the effects of SR on spinal interbody fusion surgery in an osteoporotic Sprague-Dawley rat;95 and finally, how SR causes osteophyte overgrowth in a model of early phase osteoarthritis in Dunkin Hartley albino guinea pigs.965. Vehicles for zinc and strontium delivery
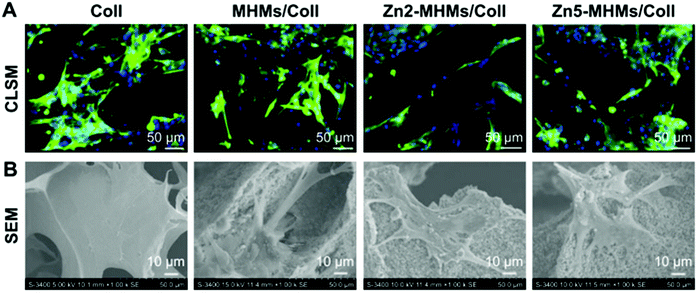
Microparticles (MPs) have been recently investigated for cartilage and bone regeneration using different materials; they also have the capacity to act as cell carriers by seeding the microparticles with cells before implantation.97 For this matter, Sr/Zn doped microspheres were evaluated using Zn doped mesoporous hydroxyapatite microspheres (MHMs) to construct a novel biomimetic scaffold with the purposes of bone regeneration in BMSCs (Fig. 5). Four scaffolds named: collagen (Coll) scaffold, MHM/Coll scaffold, Zn2-MHM/Coll scaffold, and Zn5-MHM/Coll were prepared and tested on an in vivo rat model.98 Analogously, strontium polyphosphate microparticles (Sr-a-polyP-MP) were analysed to induce mineralization of bone cells in comparison to calcium polyphosphate microparticles (Ca-a-polyP-MP) and β-tricalcium phosphate (β-TCP), which were applied to Sarcoma Osteogenic cells (SAOS-2) and hMSC cells. These MPs were encapsulated in PGLA microspheres (MS), which leaves them with three samples to contrast: β-TCP-MS, Ca-a-polyP-MS,99 and lastly, SR loaded PGLA microspheres (PM), where PGLA was degraded by hydrolysis into lactic and glycolic acid, which can develop into an inflammatory response. To this end, three samples were fabricated and contrasted: PM, SR-PM and SR-PM loaded with hydroxyapatite nanoparticles (SR-PM-HA), and MC3T3E1 cells were treated with them believing the microspheres with HA can neutralize the acidity of the lactic and glycolic acid.1006. Composite materials loaded with zinc and strontium
As previously mentioned, Zn is crucial for growth and development. Although the pathways are not completely recognized, it has been shown that Zn has a protective effect on bone loss by enhancing bone formation and restraining bone resorption, having a therapeutic effect on bone disorders.101 Additionally, the use of electromagnetic fields (EMFs) has shown curative effects in osteoporosis treatments.102 Fathi E. et al. attempt to accomplish intensified osteogenic differentiation by exposing samples to EMF whilst treating with ZnSO4. The samples used were the control (neither ZnSO4 nor EMF exposure), EMF (without ZnSO4 yet 50 Hz, 20 mT EMF exposure), ZnSO4 (without EMF exposure yet 0.432 μg ml−1 ZnSO4) and lastly EMF + ZnSO4 (0.432 μg ml−1 ZnSO4 and 50 Hz, 20 mT EMF exposure) and they were applied to ADSC cells.103The potential characteristics in osteoinduction of zinc silicate have been studied using different methods. One uses a reduced graphene oxide/zinc silicate/calcium silicate (RGO/ZS/CS) electroconductive biocomposite by spin-coating zinc silicate and strontium alginate (SA) onto a calcium silicate disk (ZS/CS disk), while simultaneously reducing graphite into reduced graphene oxide (RGO) and creating an RGO/SA suspension. Once everything was prepared, RGO/SA was spin coated onto ZN/CS disks and annealed at several temperatures, achieving RGO/ZN/CS biocomposites, which were further applied to BMSC cells.104 Another uses willemite (ZnSiO4) coated polymeric fibrous polyethersulphone–polyethylene glycol (PES–PEG) bioceramic nanoparticles. The PEG–PES fibers were fabricated by electrospinning followed by the immersion of the fibers into a 1 wt(%) Zn2SO4 solution and they were used to treat SAOS and hMSC cells.105
Additionally, particular methods were carried out in four of the analysed studies: Sr doped octacalcium phosphate (OCP) since it has been suggested that OCP is a precursor to biological apatite crystals,106 where several concentrations of Sr were prepared (1Sr-OCP, 5Sr-OCP, 10Sr-OCP, 15Sr-OCP, 25Sr-OCP) by introducing Sr into OCP by substitution of Ca2+ and testing whether there was an induction of osteogenic gene expression in BMSCs;107 Sr doped hydroxyapatite membranes (SrHA), analysing three different samples: collagen membrane, Sr10 (10 mg ml−1) and Sr20 (20 mg ml−1) with BMSCs and further along implanted into Wistar rats;108 a β-glycerophosphate (β-GP) based gel known for its thermo-inducing gelation method, where three samples were compared: control (CS/G/β-GP), Sr doped (CS/G/β-GP/Sr) and tricalcium phosphate doped (CS/G/β-GP/TC), believing it would induce osteoblast differentiation from stem cells extracted from human exfoliated deciduous teeth (SHEDs);109 pure Sr folate salts obtained from folic acid and strontium chloride solutions comparing the results with calcium folate and folic acid in HOBs cells.110 The effects of pure Zn (99.9998%) and Mg alloy ALZ31 (Mg96/Al3/Zn1) rods were evaluated111 and so was the direct supplementation of SW1353 chondrosarcoma cells;112 the effect of strontium citrate was analysed on bone consolidation during mandibular distraction in New Zealand white rabbits;113 and ultimately, an injectable Sr hybrid system composed of a RGD-alginate hydrogel cross-linked with Sr and reinforced with Sr doped hydroxyapatite microspheres was investigated in Wistar Han rats.114
7. Discussion and future perspectives
Throughout the article, the latest advances in strontium and zinc based regenerative therapies have been reviewed. These two cations have attracted the attention of several research groups due to their safety, easy accessibility and high stability conferring regenerative characteristics to biomaterials. Sixty-eight articles which performed an investigation using Sr and/or Zn regenerative therapies were analysed, within which a great variety of in vitro and in vivo studies have been conducted in order to test new therapies and bone osteogenic strategies. As shown in Fig. S1 (ESI†), these papers were organized into five main groups according to the biomaterial that was used in each study. The studies performed in vitro were roughly composed of half performed on animal cell lines, and the other half on human cell lines. Most of them analysed bone formation by evaluating: cell morphology and proliferation; differentiation into osteoblasts; expression of osteoblastic-related genes such as: alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx2), osteocalcin (Bglap), collagen type I α-chain (COL1), osteopontin (Spp1) and bone morphogenetic protein 2 (BMP2) by carrying out a PCR; and Ca deposition in the ECM. The inhibition of bone resorption was analysed by: inhibition of osteoclast formation (osteoclastogenesis) and inhibition of differentiation into osteoclasts.On the other hand, the studies performed in vivo were composed of only two papers performing an in vivo human study. Amongst the twenty-seven articles found, eleven carried out the experiment on rats; nine utilized rabbits; three used mice; one used a guinea pig and lastly, another one worked with a sheep. The majority concluded with promising results by analysing: bone repair, new and/or increased bone formation, biocompatibility, osteoconductivity, reduced risk of fracture and augmented tissue mineralization. In addition, most of the studies analysed the effect of Sr and/or Zn in femur or tibia areas in animals. Furthermore, excepting three articles in which two used Zn and the other one combined Sr and Zn; twenty-four of the studies performed in vivo used Sr as the main element to promote bone regeneration in osteoporotic – mostly induced – animal models. All these studies are summarized in Table 1 which helps to easily identify the method applied, element evaluated, tissues/cells targeted, and main results achieved from each study.
| Material | Method | Elementa | Ref. | In vitro cell lines | In vivo species | Target | Results |
|---|---|---|---|---|---|---|---|
| a Element of interest to this review.Abbreviations: Li, lithium; CS, calcium sulphate; PMMA, poly(methylmethacrylate); SrBG, strontium-containing borate bioactive glass; IGF-1, insulin growth factor; Sr–HT–gahnite, strontium–hardysonite–gahnite; Col-1, type I collagen; Mg–Zr–Sr, magnesium–zirconia–strontium; BMD, bone mass density; SrO, strontium oxide; Ca, calcium. N.A. Not applicable. | |||||||
| Scaffold | Sr-substituted bioactive glass | Sr | Santocildes-Romero41 | BMSCs | N.A. | N.A. | Osteoblastic differentiation |
| SrO-substituted bioactive glass | Sr | Moghanian42 | MC3T3-E1 | N.A. | N.A. | Higher ALP activity and fewer dead cells | |
| Sr and Li bioactive glass | Sr | Khan43 | NIH3T3 | New Zealand white rabbit | Femur | In vitro: osteoblast/osteoclast proliferation | |
| In vivo: acceleration of early-stage bone formation | |||||||
| Sr-CS bioactive graft | Sr | Jun44 | MC3T3-E1 | New Zealand white rabbit | Femur | In vitro: osteoblastic proliferation | |
| In vivo: better capacity for the repair of critical bone defects | |||||||
| Sr-phosphate bioactive glass | Sr | Stefanic46 | hMSCs | N.A. | N.A. | Osteogc differentiation | |
| Sr and Ca-phosphate bioactive glass | Sr | Al qaysi47 | MG-63 | N.A. | N.A. | Enhanced ALP activity and osteoinduction | |
| Sr and Co-bioactive glass | Sr | Kargozar48 | HUVECs SAOS-2 | N.A. | N.A. | Promoted osteogenesis and angiogenesis | |
| Zn and Sr based phosphate glass | Sr + Zn | Alqaysi49 | MG-63 | N.A. | N.A. | Higher ALP activity and calcium deposition. | |
| Sr and Cu binary doped nanofibers | Sr | Weng53 | ADSCs RAW264.7 | N.A. | N.A. | Enhanced osteogenesis and suppressed osteoclastogenesis | |
| Sr-nanofibers | Sr | Meka54 | hMSC | N.A. | N.A. | Enhanced expression of osteogenic related genes | |
| Sr-phosphate nanofibers | Sr | Su55 | SHEDs | N.A. | N.A. | Enhanced ALP activity and calcium deposition | |
| PMMA bone cement + SrBG bioactive glass | Sr | Cui59 | MC3T3-E1 | Sprague-Dawley rat | Tibia | In vitro: higher ALP activity and osteoblast attachment | |
| In vivo: stimulated new-bone formation | |||||||
| Sr substituted bone cement | Sr | Montesi60 | MC3T3-E1 | N.A. | N.A. | Induction of osteogenic gene expression | |
| RAW264.7 | |||||||
| SrO-biosilicate scaffold | Sr | Shuai63 | MG-63 | N.A. | N.A. | Enhanced cell adhesion, proliferation and ALP activity | |
| Zn2SiO4 coated scaffold | Zn | Bageshlooy Afshar64 | ADSCs | N.A. | N.A. | Higher ALP activity | |
| Higher calcium content | |||||||
| Osteogenic differentiation | |||||||
| Sr doped porcine derived scaffolds | Sr | Cheng65 | hMSCs | N.A. | N.A. | Osteogenic differentiation and higher ALP activity | |
| Sr doped hybrid scaffold | Sr | John66 | HOBs | N.A. | N.A. | Osteoblastic proliferation and growth | |
| Sr folate loaded biohydrid scaffolds | Sr | Martin del Campo67 | HDPSCs | Wistar rat | Skull | In vitro: osteogenic related gene expression and differentiation | |
| In vivo: increased new bone formation | |||||||
| Mg–Zn–Ca alloy composite scaffolds | Zn | Guan68 | WJCs | N.A. | N.A. | High ALP activity | |
| Sr–HT–gahnite scaffolds | Sr + Zn | Graney71 | hPBM | N.A. | N.A. | Modulation of macrophage behavior | |
| Sr + Zn | Wang72 | ASCs HUVEC | N.A. | N.A. | Osteogenic and angiogenic proliferation and differentiation. | ||
| Sr + Zn | Roohani-Esfahani73 | HOBs | New Zealand white rabbit | Radius | In vitro: enhanced osteogenic-related gene expression | ||
| In vivo: regeneration of bone in rabbit radial defects with no adverse effects | |||||||
| Sr-bioactive glass | Sr | Zhang45 | N.A. | Wistar rat | Jaw | Increased bone regeneration in periodontal tissues | |
| Sr-bone cement | Sr | Rohnke61 | N.A. | Sprague-Dawley rat | Femur | Assisted new bone formation | |
| IGF-1 + Zn loaded bone cements | Zn | Vahabzadeh62 | N.A. | New Zealand white rabbit | Distal femur | Accelerated early bone formation | |
| Sr-hydroxyapatite | Sr | Machado56 | N.A. | Sheep | Tibia | Biocompatibility and osteoconductivity | |
| Sr-hydroxyapatite | Sr | Ehret57 | N.A. | Mouse | Subcutaneous implant | Enhanced tissue mineralization and bone formation | |
| Col-1 coating on Mg–Zr–Sr implants | Sr | Mushahary69 | N.A. | New Zealand white rabbit | Femur | Bone formation and lower bone turnover | |
| Sr-apatite/polylactide composites | Sr | Luo70 | N.A. | New Zealand white rabbit | Distal femur | Increased bone formation | |
| Titanium surface | SrTiO3/TiO2 nanotubes | Sr | Yin76 | RAW264.7 | N.A. | N.A. | Osteogenic differentiation |
| Sr loaded titania nanotubes | Sr | Mi78 | RAW264.7 | Sprague-Dawley rat | Tibia | In vitro: osteoclast differentiation inhibition | |
| In vivo: prevented bone loss | |||||||
| Sr and Ca co-doped titanium oxides | Sr | Yuan79 | BMSC RAW264.7 | C57BL/6 | Dorsal area | In vitro: osteogenic differentiation | |
| Mouse | In vivo: bone formation | ||||||
| Sr ions on Ca and P-containing titanium oxides | Sr | Sato80 | MC3T3E1 | N.A. | N.A. | Cell osteogenic differentiation and calcification | |
| Biomimetic OCP containing Sr | Sr | Birgani82 | hMSCs | N.A. | N.A. | Osteogenic differentiation | |
| Sr–sodium alginate coating on a titanium surface | Sr | Jia87 | MG-63 | N.A. | N.A. | Cell adhesion and proliferation Enhanced ALP activity | |
| SrO–titanium surface | Sr | Fan83 | N.A. | New Zealand white rabbit | Tibia Femur | Promoted effect on new bone formation | |
| Sr + Mg bioactive titanium metal | Sr | Okuzu84 | N.A. | Japanese white rabbit | Tibia | Early bone bonding | |
| Strontium ranelate | Strontium ranelate | Sr | Shi89 | N.A. | Mouse | Femur | Reduced fracture incidence |
| Strontium ranelate | Sr | Pan90 | N.A. | Human | Jaw | Recovery of bone turnover and BMD | |
| Strontium ranelate | Sr | Amaral91 | N.A. | Lewis rat | Jaw | Anticipated process of neoformation and bone maturation | |
| Strontium ranelate + insulin | Sr | Sava92 | N.A. | Wistar rat | Tibia | Bone regeneration | |
| Strontium ranelate | Sr | Brun93 | N.A. | Human | General bone mineral density | Increased BMD and bone formation markers, decreased bone resorption | |
| Strontium ranelate | Sr | Lavet94 | N.A. | Sprague-Dawley rat | Tibia | Normalized bone formation while reduced bone resorption | |
| Strontium ranelate | Sr | Tsai95 | N.A. | Sprague-Dawley rat | Spine | Successful fusion union | |
| Strontium ranelate | Sr | Chu96 | N.A. | Dunkin Hartley albino guinea pigs | Knee | Enhanced bone formation | |
| Calcium channel activation | Sr | Fernández115 | MC3T3E1 | N.A. | N.A. | Osteoblast protection against AGE | |
| Microparticles | SR-PM-HA | Sr | Mao100 | MC3T3E1 | N.A. | N.A. | Osteogenic differentiation |
| Higher ALP activity | |||||||
| Zn-doped mesoporous HA microspheres | Zn | Yu98 | BMSCs | Rat | Femur | In vitro: osteogenic differentiation | |
| In vivo: rapid bone formation | |||||||
| Sr polyphosphate microparticles | Sr | Muller99 | SAOS-2 hMSCs | N.A. | N.A. | Enhanced ALP and BMP-2 activity | |
| Zinc composites | Electromagnetic field | Zn | Fathi103 | ADSCs | N.A. | N.A. | Higher ALP activity and calcium levels |
| (RGO/ZS/CS) biocomposite | Zn | Xiong104 | BMSC RAW264.7 | N.A. | N.A. | Enhanced ALP activity | |
| Suppressed osteoclastic differentiation | |||||||
| PES–PEG electrospun fibrous composites | Zn | Amiri105 | hMSCs | N.A. | N.A. | Enhanced ALP activity and calcium deposition | |
| Other methods | Sr-OCP | Sr | Shi107 | BMSC | N.A. | N.A. | Induction of osteogenic gene expression |
| Sr hydroxyapatite membrane | Sr | Hao108 | BMSC | Wistar rat | Skull | In vitro: osteoblastic cell growth and differentiation | |
| In vivo: stimulated bone formation at earlier stage | |||||||
| Sr phosphate chitosan-based hydrogels | Sr | Su109 | SHEDs | N.A. | N.A. | Osteoblastic differentiation. Increased gene expression | |
| Sr and calcium-based folates | Sr | Rojo110 | HOBs | N.A. | N.A. | Overexpression of ALP activity | |
| Zn and Mg biomaterials | Zn | Zhu111 | hMSCs | N.A. | N.A. | Mineralization of ECM and high ALP activity | |
| Strontium citrate | Sr | Taylor113 | N.A. | New Zealand white rabbit | Jaw | Accelerated new bone formation | |
| Sr local delivery injectable hybrid | Sr | Henriques Lourenço114 | N.A. | Wistar Han rat | Knee | Increased bone formation | |
As the results showed for the in vitro studies, it is evident that Sr and Zn can not only induce osteogenesis and inhibit osteoclast formation, but also promote various processes such as calcification of the bone matrix and enhance osteogenic-related genes that contribute to bone healing. The majority of the materials analysed resulted in bone formation or inhibition of bone resorption, obtaining as results: induction/enhancement of ALP activity of osteogenic related genes; osteoblastic differentiation or proliferation e.g. osteogenesis, angiogenesis, bone formation and osteoblastic formation among others; calcium deposition or mineralization of the ECM was also evaluated; inhibition of osteoclast formation or bone resorption; osteoblast attachment or enhanced cell adhesion; modulation of macrophage response or immunomodulation; and osteoblast protection against AGE.
A few drawbacks have been identified concerning vehiculation of divalent cations into biomaterials. Current evidence affirms that strontium ranelate mediates an uncoupling in bone turnover since it enhances osteogenesis and osteoblast activity while decreasing osteoclast differentiation and function;116 but also that it has serious side effects such as memory loss or increased risk of venous thromboembolism.15 However, new investigations substituting ranelic acid for vitamin-B derivatives of Sr or Zn are being recently described with very promising characteristics.117
Biocompatibility is crucial in terms of regenerative therapies; most of the studies were carried out using Zn and/or Sr doped scaffolds (bioactive glasses, nanofibers…), Ti surfaces and Zn compounds, which appear to have exceptional biocompatibility. A Sr and Zn co-doped scaffold even achieved modulation of macrophage behaviour,71 implying no rejection from the cells. The Sr and/or Zn doped or loaded materials were able to stimulate a specific cellular response, thus exhibiting promising features to assist new bone formation, particularly relevant in the case of bone defects due to metabolic diseases, such as osteoporosis. Nonetheless, the weakness of the in vitro studies is that the data is complex to extrapolate due to the differences in the metabolism and drug development in distinct species and the different conditions of the surroundings when working in vivo.
On the other hand, the results from the in vivo studies also confirm that the addition of Sr and/or Zn can alter the physicomechanical characteristics and effectively enhance early stage in vivo osseointegration and recovery from bone turnover as well as tissue mineralization. The majority of the papers evaluated acceleration of early-stage bone formation/increased bone regeneration, yet some other results were pursued: recovery from bone resorption/turnover; augmented bone mass density (BMD); biocompatibility and osteoconductivity; enhanced tissue mineralization; decreased fracture incidence; early bone bonding, and prevented bone loss.
It should be noted that the one Sr and Zn co-doped scaffold concluded with the regeneration of bone in rabbit radial defects with no adverse effects.73 Most of the biomaterials used demonstrated proper setting times and high mechanical strength, and released ion products (such as Ca, Sr, Zn and P), imparting bioactivity to the composite cement and confirming the cytocompatibility of the composites. Nevertheless, only two studies were performed in humans, thus the same problem is faced: differences in the metabolism and drug development in distinct species. Moreover, the experiments taking place in humans were performed with SR which is already being used as an anti-osteoporotic treatment, meaning that the novel and innovative proposals of regenerative therapies are yet to be tested in humans.
The obtained results of the in vitro cell culture and the in vivo studies suggest that Sr and/or Zn have the potential to address the unmet need for bone substitutes that can be used in load and partial load-bearing sites in orthopedic applications as well as in osteoporotic defects, inducing bone formation to a level comparable to healthy animals at an ectopic site. In addition, the cellular mechanisms by which Sr and Zn induce osteogenesis and inhibit osteoclastogenesis are mediated through the RANKL/RANK system by suppressing RANKL-stimulation in osteoclastogenesis. From the results of the aforesaid studies, it can also be deduced that the osteogenic-induced effect carried out by the addition of Sr and/or Zn is dose-dependent, meaning that lower doses than the established may not be able to provide the desired effects and higher doses can have detrimental effects.
The role of Zn in cartilage protection promoting healthy collagen II production and conformation has been proven.36 In addition, some authors have demonstrated that zinc can protect against the action of pro-inflammatory cytokines (1L-1β) through the activation of antioxidant genes preventing osteoarthritis. Controversially, elevated Zn levels lead to the activation of biochemical pathways in chondrocytes that result in catabolic function in chondrocytes upregulating the matrix metalloproteinases enzymes, thus demonstrating anti-inflammatory effects of Zn sequestrating regenerative approaches.118 From the results of the aforesaid studies, it can also be deduced that the osteogenic-induced effect carried out by the addition of both Sr and/or Zn is dose-dependent, meaning that lower doses than the established may not be able to provide the desired effects and higher doses can have detrimental effects.
In conclusion, current investigations demonstrate that strontium and zinc induce human stem cell differentiation towards cartilage and bone like phenotypes in vitro and biomaterials containing such enhanced their performance both in vitro and in vivo, inducing new bone and cartilage tissue formation and inhibiting tissue resorption. Thus, novel organic and inorganic bioactive derivatives of strontium and zinc are in continuous development in order to overcome some limitations related to existing drugs and delivery systems. These compounds, together with new insights about the use of strontium and zinc-based biomaterials, confirm their potential to inspire advanced tissue engineering therapies for emerging regenerative medicine.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish Program MICINN (MAT201573656-JIN)References
- R. Florencio-Silva, G. Sasso, E. Sasso-Cerri, M. J. Simões and P. S. Cerri, BioMed Res. Int., 2015, 2015, 1–17 CrossRef PubMed.
- G. A. Rodan and T. J. Martin, Science, 2000, 289, 1508–1514 CrossRef CAS PubMed.
- A. D. Woolf and B. Pfleger, Bull. W. H. O., 2003, 81, 646–656 Search PubMed.
- http://appsso.eurostat.ec.europa.eu/nui/submitViewTableAction.do .
- A. S. Mao and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 14452–14459 CrossRef CAS PubMed.
- P. V. Giannoudis, G. M. Calori, T. Bégué and G. Schmidmaier, Injury, 2015, 46, S1–S2 CrossRef PubMed.
- https://www.iofbonehealth.org/what-is-osteoporosis.com .
- N. Neves, D. Linhares, G. Costa, C. C. Ribeiro and M. A. Barbosa, Bone Jt. Res., 2017, 6, 366–375 CrossRef CAS PubMed.
- S. L. Teitelbaum, Science, 2000, 289, 1504–1508 CrossRef CAS PubMed.
- U.S. Department of Health and Human Services, Bone Health and Osteoporosis: A Report of the Surgeon General, U.S. Department of Health and Human Services, Office of the Surgeon General, Rockville, MD, 2004.
- A. I. Gogakos, M. S. Cheung, J. H. D. Bassett and G. R. Williams, Expert Rev. Endocrinol. Metab., 2009, 4, 639–650 CrossRef CAS PubMed.
- S. C. Manolagas, Maturitas, 2014, 78, 233–237 CrossRef CAS PubMed.
- J. Kim and N. Kim, Chonnam Med. J., 2016, 52, 12–17 CrossRef CAS PubMed.
- Q. Bian, Y.-J. Wang, S. Liu and Y.-P. Li, Biochemistry, 2012, 1, 639–650 Search PubMed.
- C. McGreevy and D. Williams, Ther. Adv. Drug Saf., 2011, 2, 159–172 CrossRef PubMed.
- C. Mason and P. Dunnill, Regener. Med., 2008, 3, 1–5 CrossRef PubMed.
- Y. Tabata, J. R. Soc., Interface, 2009, 6, S311–S324 CrossRef CAS PubMed.
- F. Colombo, G. Sampogna, G. Cocozza, S. Y. Guraya and A. Forgione, J. Microsc. Ultrastruct., 2017, 5, 1–8 CrossRef PubMed.
- G. Sampogna, S. Y. Guraya and A. Forgione, J. Microsc. Ultrastruct., 2015, 3, 101–107 CrossRef PubMed.
- S. Pors Nielsen, Bone, 2004, 35, 583–588 CrossRef PubMed.
- E. Abdullah, A. Idris and A. Saparon, ARPN J. Eng. Appl. Sci., 2017, 12, 3218–3221 Search PubMed.
- J. M. Fernández, M. S. Molinuevo, A. D. McCarthy and A. M. Cortizo, Biometals, 2014, 27, 601–607 CrossRef PubMed.
- W. Querido, A. L. Rossi and M. Farina, Micron, 2016, 80, 122–134 CrossRef CAS PubMed.
- S. Pasqualetti, G. Banfi and M. Mariotti, J. Trace Elem. Med. Biol., 2013, 27, 375–379 CrossRef CAS PubMed.
- G. M. Blake and I. Fogelman, Clin. Interv. Aging, 2006, 1, 367–375 CrossRef CAS PubMed.
- H. Y. Lee, D. Lie, K. S. Lim, T. Thirumoorthy and S. M. Pang, Osteoporos. Int., 2009, 20, 161–162 CrossRef CAS PubMed.
- A. Aimaiti, A. Maimaitiyiming, X. Boyong, K. Aji, C. Li and L. Cui, Stem Cell Res. Ther., 2017, 8, 282 CrossRef PubMed.
- J. Zarins, M. Pilmane, E. Sidhom and I. Salma, Acta Chir. Latviensis, 2017, 17–23 Search PubMed.
- C. Cai, P. Lin, H. Zhu, J. K. Ko, M. Hwang, T. Tan, Z. Pan, I. Korichneva and J. Ma, J. Biol. Chem., 2015, 290, 13830–13839 CrossRef CAS PubMed.
- N. M. Lowe, W. D. Fraser and M. J. Jackson, Proc. Nutr. Soc., 2002, 61, 181–185 CrossRef CAS PubMed.
- J. Mayer, Postgrad. Med., 1964, 35, 206–209 CrossRef CAS PubMed.
- T. Fukada, S. Yamasaki, K. Nishida, M. Murakami and T. Hirano, J. Biol. Inorg. Chem., 2011, 16, 1123–1134 CrossRef CAS PubMed.
- C. R. Flannery, C. B. Little, C. E. Hughes and B. Caterson, Int. J. Exp. Pathol., 2000, 81, 1–29 CrossRef.
- M. Demoor, D. Ollitrault, T. Gomez-Leduc, M. Bouyoucef, M. Hervieu, H. Fabre, J. Lafont, J. M. Denoix, F. Audigié, F. Mallein-Gerin, F. Legendre and P. Galera, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 2414–2440 CrossRef CAS PubMed.
- L. Troeberg and H. Nagase, Biochim. Biophys. Acta, Proteins Proteomics, 2012, 1824, 133–145 CrossRef CAS PubMed.
- K. Rosenberg, H. Olsson, M. Morgelin and D. Heinegard, J. Biol. Chem., 1998, 273, 20397–20403 CrossRef CAS PubMed.
- A. Rahmani Del Bakhshayesh, N. Annabi, R. Khalilov, A. Akbarzadeh, M. Samiei, E. Alizadeh, M. Alizadeh-Ghodsi, S. Davaran and A. Montaseri, Artif. Cells, Nanomed. Biotechnol., 2017, 1–15 Search PubMed.
- L. Rojo del Olmo, Adv. Exp. Med. Biol., 2018, 1059, 301–313 CrossRef PubMed.
- L. Rojo, S. Radley-Searle, M. Fernandez-Gutierrez, L. M. Rodriguez-Lorenzo, C. Abradelo, S. Deb and J. San Roman, J. Mater. Chem. B, 2015, 3, 2708–2713 RSC.
- M. N. Rahaman, D. E. Day, B. Sonny Bal, Q. Fu, S. B. Jung, L. F. Bonewald and A. P. Tomsia, Acta Biomater., 2011, 7, 2355–2373 CrossRef CAS PubMed.
- M. E. Santocildes-Romero, A. Crawford, P. V. Hatton, R. L. Goodchild, I. M. Reaney and C. A. Miller, J. Tissue Eng. Regener. Med., 2015, 9, 619–631 CrossRef CAS PubMed.
- A. Moghanian, S. Firoozi and M. Tahriri, Ceram. Int., 2017, 43, 14880–14890 CrossRef CAS.
- P. K. Khan, A. Mahato, B. Kundu, S. K. Nandi, P. Mukherjee, S. Datta, S. Sarkar, J. Mukherjee, S. Nath, V. K. Balla and C. Mandal, Sci. Rep., 2016, 6, 32964 CrossRef CAS PubMed.
- W. Jun, W. Peng, J. Dianming, L. Hong, L. Cong, L. Xing, Q. Xiangyang, C. Yujiang and L. Ming, RSC Adv., 2017, 7, 54306–54312 RSC.
- Y. Zhang, L. Wei, C. Wu and R. J. Miron, PLoS One, 2014, 9, 1–6 CAS.
- M. Stefanic, M. Peroglio, A.-M. Stanciuc, G. C. Machado, I. Campbell, M. M. Kržmanc, M. Alini and X. Zhang, J. Eur. Ceram. Soc., 2018, 887–897 CrossRef CAS.
- M. Al Qaysi, N. J. Walters, F. Foroutan, G. J. Owens, H.-W. Kim, R. Shah and J. C. Knowles, J. Biomater. Appl., 2015, 30, 300–310 CrossRef CAS PubMed.
- S. Kargozar, N. Lotfibakhshaiesh, J. Ai, M. Mozafari, P. Brouki Milan, S. Hamzehlou, M. Barati, F. Baino, R. G. Hill and M. T. Joghataei, Acta Biomater., 2017, 58, 502–514 CrossRef CAS PubMed.
- M. Alqaysi, A. Aldaadaa, N. Mordan, R. Shah and J. C. Knowles, Biomed. Mater., 2017, 12, 65011 CrossRef PubMed.
- G. Kaur, G. Pickrell, N. Sriranganathan, V. Kumar and D. Homa, J. Biomed. Mater. Res., Part B, 2016, 104, 1248–1275 CrossRef CAS PubMed.
- X. Feng, Curr. Chem. Biol., 2009, 3, 189–196 CAS.
- J. M. Holzwarth and P. X. Ma, Biomaterials, 2011, 32, 9622–9629 CrossRef CAS PubMed.
- L. Weng, S. K. Boda, M. J. Teusink, F. D. Shuler, X. Li and J. Xie, ACS Appl. Mater. Interfaces, 2017, 9, 24484–24496 CrossRef CAS PubMed.
- S. R. K. Meka, S. Jain and K. Chatterjee, Colloids Surf., B, 2016, 146, 649–656 CrossRef CAS PubMed.
- W. Su, P. Wu and T. Huang, Mater. Sci. Eng., C, 2015, 46, 427–434 CrossRef CAS PubMed.
- C. P. G. Machado, S. C. Sartoretto, A. T. N. N. Alves, I. B. C. Lima, A. M. Rossi, J. M. Granjeiro and M. D. Calasans-Maia, Braz. Oral Res., 2016, 30, 1–11 Search PubMed.
- C. Ehret, R. Aid-Launais, T. Sagardoy, R. Siadous, R. Bareille, S. Rey, S. Pechev, L. Etienne, J. Kalisky, E. De Mones, D. Letourneur and J. A. Vilamitjana, PLoS One, 2017, 12, 1–21 CrossRef PubMed.
- L. Rojo, B. Vazquez, S. Deb and J. San Román, Acta Biomater., 2009, 5, 1616–1625 CrossRef CAS PubMed.
- X. Cui, C. Huang, M. Zhang, C. Ruan, S. Peng, L. Li, W. Liu, T. Wang, B. Li, W. Huang, M. N. Rahaman, W. W. Lu and H. Pan, J. R. Soc., Interface, 2017, 14, 20161057 CrossRef PubMed.
- M. Montesi, S. Panseri, M. Dapporto, A. Tampieri and S. Sprio, PLoS One, 2017, 12, 1–13 CrossRef PubMed.
- M. Rohnke, S. Pfitzenreuter, B. Mogwitz, A. Henß, J. Thomas, D. Bieberstein, T. Gemming, S. K. Otto, S. Ray, M. Schumacher, M. Gelinsky and V. Alt, J. Controlled Release, 2017, 262, 159–169 CrossRef CAS PubMed.
- S. Vahabzadeh, A. Bandyopadhyay, S. Bose, R. Mandal and S. K. Nandi, Integr. Biol., 2015, 7, 1561–1573 CrossRef CAS PubMed.
- C. Shuai, H. Sun, P. Wu, C. Gao, Y. Yang, W. Guo, D. Yang, F. Xu, P. Feng and S. Peng, RSC Adv., 2017, 7, 21749–21757 RSC.
- B. Bageshlooyafshar, S. Vakilian, M. Kehtari, T. Eslami-Arshaghi, F. Rafeie, R. Ramezanifard, R. Rahchamani, A. Mohammadi-Sangcheshmeh, Y. Mostafaloo and E. Seyedjafari, Res. Vet. Sci., 2017 DOI:10.1016/j.rvsc.2017.09.015.
- A. D. Cheng, Q. Liang and Y. Li, Colloids Surf., B, 2018, 162, 279–287 CrossRef PubMed.
- Ł. John, M. Podg, J. Nedelec, Ł. Cwynar and P. Dzięgiel, Mater. Sci. Eng., C, 2016, 68, 117–127 CrossRef PubMed.
- M. Martin-del-Campo, R. Rosales-Ibañez, K. Alvarado, J. G. Sampedro, C. A. Garcia-Sepulveda, S. Deb, J. San Román and L. Rojo, Biomater. Sci., 2016, 4, 1596–1604 RSC.
- F. Guan, S. Ma, X. Shi, X. Ma, L. Chi, S. Liang, Y. Cui, Z. Wang, N. Yao, S. Guan and B. Yang, Sci. China: Life Sci., 2014, 57, 181–187 CrossRef CAS PubMed.
- D. Mushahary, C. Wen, J. M. Kumar, R. Sravanthi, P. Hodgson, G. Pande and Y. Li, Clin. Oral. Implants Res., 2016, 27, e15–e24 CrossRef PubMed.
- X. Luo, D. Barbieri, R. Duan, H. Yuan and J. D. Bruijn, Acta Biomater., 2015, 26, 331–337 CrossRef CAS PubMed.
- P. L. Graney, S.-I. Roohani-Esfahani, H. Zreiqat and K. L. Spiller, J. R. Soc., Interface, 2016, 13, 20160346 CrossRef PubMed.
- G. Wang, S.-I. Roohani-Esfahani, W. Zhang, K. Lv, G. Yang, X. Ding, D. Zou, D. Cui, H. Zreiqat and X. Jiang, Sci. Rep., 2017, 7, 41135 CrossRef CAS PubMed.
- S. I. Roohani-Esfahani, C. R. Dunstan, J. J. Li, Z. Lu, B. Davies, S. Pearce, J. Field, R. Williams and H. Zreiqat, Acta Biomater., 2013, 9, 7014–7024 CrossRef CAS PubMed.
- J. J. Li, M. Ebied, J. Xu and H. Zreiqat, Adv. Healthcare Mater., 2018, 7, 1701061 CrossRef PubMed.
- D. Losic, M. S. Aw, A. Santos, K. Gulati and M. Bariana, Expert Opin. Drug Delivery, 2015, 12, 103–127 CrossRef CAS PubMed.
- L. Yin, J. Zhou, L. Gao, C. Zhao, J. Chen, X. Lu, J. Wang, J. Weng and B. Feng, Surf. Coat. Technol., 2017, 330, 121–130 CrossRef CAS.
- B. Palla-Rubio, N. Araújo-Gomes, M. Fernández-Gutiérrez, L. Rojo, J. Suay, M. Gurruchaga and I. Goñi, Carbohydr. Polym., 2019, 203, 331–341 CrossRef CAS PubMed.
- B. Mi, W. Xiong, N. Xu, H. Guan, Z. Fang, H. Liao, Y. Zhang, B. Gao, X. Xiao, J. Fu and F. Li, Sci. Rep., 2017, 7, 2328 CrossRef PubMed.
- X. Yuan, H. Cao, J. Wang, K. Tang, B. Li, Y. Zhao, M. Cheng, H. Qin, X. Liu and X. Zhang, Front. Immunol., 2017, 8, 1196 CrossRef PubMed.
- M. Sato, P. Chen, Y. Tsutsumi, M. Shiota, T. Hanawa and S. Kasugai, Dent. Mater. J., 2016, 35, 627–634 CrossRef CAS PubMed.
- L. H. Li, H. W. Kim, S. H. Lee, Y. M. Kong and H. E. Kim, J. Biomed. Mater. Res., Part A, 2005, 73, 48–54 CrossRef PubMed.
- Z. T. Birgani, A. Malhotra, C. A. van Blitterswijk and P. Habibovic, J. Biomed. Mater. Res., Part A, 2016, 104, 1946–1960 CrossRef CAS PubMed.
- Y. P. Fan, X. Y. Chen, Y. Chen, G. L. Yang, H. M. Wang and F. M. He, Clin. Oral. Implants Res., 2017, 28, 911–919 CrossRef PubMed.
- Y. Okuzu, S. Fujibayashi, S. Yamaguchi, K. Yamamoto, T. Shimizu, T. Sono, K. Goto, B. Otsuki, T. Matsushita, T. Kokubo and S. Matsuda, Acta Biomater., 2017, 63, 383–392 CrossRef CAS PubMed.
- J. Venkatesan, R. Nithya, P. N. Sudha and S. K. Kim, Role of alginate in bone tissue engineering, Elsevier Inc., 1st edn, 2014, vol. 73 Search PubMed.
- E. S. Place, L. Rojo, E. Gentleman, J. P. Sardinha and M. M. Stevens, Tissue Eng., Part A, 2011, 17, 2713–2722 CrossRef CAS PubMed.
- N. Yuan, L. Jia, Z. Geng, R. Wang, Z. Li, X. Yang, Z. Cui, S. Zhu, Y. Liang and Y. Liu, BioMed Res. Int., 2017, 2017, 9867819 Search PubMed.
- S. Lam and K. Zouzias, Consult. Pharm., 2008, 330, 1400–1401 Search PubMed.
- C. Shi, B. Hu, L. Guo, P. Cao, Y. Tian, J. Ma, Y. Chen, H. Wu, J. Hu, L. Deng, Y. Zhang and W. Yuan, J. Bone Miner. Res., 2016, 31, 1003–1014 CrossRef CAS PubMed.
- W.-L. Pan, P.-L. Chen, C.-Y. Lin, Y.-C. Pan, Y.-R. Ju, C.-P. Chan and R. W. W. Hsu, Clin. Interventions Aging, 2017, 12, 1089–1093 CrossRef PubMed.
- S. A. Amaral, I. D. G. Reis, P. A. D. Oliveira, F. O. Costa, A. M. de Goes, G. A. B. Silva and L. O. de M. Cota, Int. J. Clin. Exp. Med., 2017, 10, 10616–10624 Search PubMed.
- A. Nemtoi, V. Danila, E. Dragan, S. Paşca, A. Nemtoi, M. Constantin, A. Sava and D. Haba, Rev. Chim., 2017, 68, 693–697 CAS.
- L. R. Brun, A. M. Galich, E. Vega, H. Salerni, L. Maffei, V. Premrou, P. R. Costanzo, M. A. Sarli, P. Rey, M. S. Larroudé, M. S. Moggia, M. L. Brance and A. Sánchez, SpringerPlus, 2014, 3, 676 CrossRef PubMed.
- C. Lavet, G. Mabilleau, D. Chappard, R. Rizzoli and P. Ammann, Osteoporosis Int., 2017, 28, 3475–3487 CrossRef CAS PubMed.
- T. T. Tsai, C. L. Tai, N. Y. J. Ho, P. L. Lai, T. S. Fu, C. C. Niu, L. H. Chen and W. J. Chen, PLoS One, 2017, 12, 1–16 Search PubMed.
- J.-G. Chu, M.-W. Dai, Y. Wang, F.-M. Tian, H.-P. Song, Y.-P. Xiao, L.-T. Shao, Y.-Z. Zhang and L. Zhang, BMC Musculoskelet. Disord., 2017, 18, 78 CrossRef PubMed.
- J. Hendriks, J. Riesle and C. A. van Blitterswijk, J. Tissue Eng. Regener. Med., 2010, 4, 524–531 CrossRef PubMed.
- W. Yu, T. W. Sun, C. Qi, Z. Ding, H. Zhao, S. Zhao, Z. Shi, Y. J. Zhu, D. Chen and Y. He, Int. J. Nanomedicine, 2017, 12, 2293–2306 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, M. Ackermann, M. Neufurth, S. Wang, Q. Feng, H. C. Schröder and X. Wang, Acta Biomater., 2017, 50, 89–101 CrossRef PubMed.
- Z. Mao, Z. Fang, Y. Yang, X. Chen, Y. Wang, J. Kang, X. Qu, W. Yuan and K. Dai, RSC Adv., 2017, 7, 24607–24615 RSC.
- M. Yamaguchi, Biomed. Res. Trace Elem., 2004, 15, 9–14 CAS.
- R. Wang, H. Wu, Y. Yang and M. Song, Electromagn. Biol. Med., 2016, 35, 384–390 CrossRef PubMed.
- E. Fathi and R. Farahzadi, PLoS One, 2017, 12, 1–19 CrossRef PubMed.
- K. Xiong, T. Wu, Q. Fan, L. Chen and M. Yan, ACS Appl. Mater. Interfaces, 2017, 9, 44356–44368 CrossRef CAS PubMed.
- B. Amiri, M. Ghollasi, M. Shahrousvand, M. Kamali and A. Salimi, Differentiation, 2016, 1–11 Search PubMed.
- O. Suzuki, H. Imaizumi, S. Kamakura and T. Katagiri, Curr. Med. Chem., 2008, 15, 305–313 CrossRef CAS PubMed.
- H. Shi, X. Ye, T. Wu, J. Zhang and J. Ye, Mater. Today Chem., 2017, 5, 81–91 CrossRef.
- J. Hao, A. Acharya, K. Chen, J. Chou, S. Kasugai and N. P. Lang, Clin. Oral. Implants Res., 2015, 26, 1–7 CrossRef CAS PubMed.
- W. Su, W. Chou and C. Chou, Mater. Sci. Eng., C, 2015, 52, 46–53 CrossRef CAS PubMed.
- L. Rojo, S. Radley-Searle, M. Fernandez-Gutierrez, L. M. Rodriguez-Lorenzo, C. Abradelo, S. Deb and J. San Roman, J. Mater. Chem. B, 2015, 3, 2708–2713 RSC.
- D. Zhu, Y. Su, M. L. Young, J. Ma, Y. Zheng and L. Tang, ACS Appl. Mater. Interfaces, 2017, 9, 27453–27461 CrossRef CAS PubMed.
- E. B. Fung, J. L. Kwiatkowski, J. N. Huang, G. Gildengorin, J. C. King and E. P. Vichinsky, Am. J. Clin. Nutr., 2013, 98, 960–971 CrossRef CAS PubMed.
- B. A. Taylor, M. Bezuhly, M. Brace, M. Carter and P. Hong, Laryngoscope, 2017, 127, E212–E218 CrossRef CAS PubMed.
- A. Henriques Lourenço, N. Neves, C. Ribeiro-Machado, S. R. Sousa, M. Lamghari, C. C. Barrias, A. Trigo Cabral, M. A. Barbosa and C. C. Ribeiro, Sci. Rep., 2017, 7, 5098 CrossRef PubMed.
- J. M. Fernández, M. S. Molinuevo, C. Sedlinsky, L. Schurman, A. M. Cortizo and A. D. McCarthy, Eur. J. Pharmacol., 2013, 706, 41–47 CrossRef PubMed.
- P. J. Marie, M. Hott, D. Modrowski, C. De Pollak, J. Guillemain, P. Deloffre and Y. Tsouderos, J. Bone Miner. Res., 1993, 8, 607–615 CrossRef CAS PubMed.
- D. Fernández-Villa, M. Jiménez Gómez-Lavín, C. Abradelo, J. San Román and L. Rojo, Int. J. Mol. Sci., 2018, 19, 4068 CrossRef PubMed.
- P. Suárez, L. Rojo, Á. González-Gómez and J. S. Román, Macromol. Biosci., 2013, 13, 1174–1184 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1. Flow diagram showing the screening process carried out in this review. See DOI: 10.1039/c8tb02738b |
| This journal is © The Royal Society of Chemistry 2019 |