Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications
Abstract
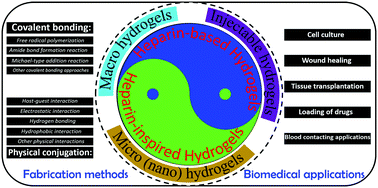
Heparin is the highest negatively charged biomolecule, which is a polysaccharide belonging to the glycosaminoglycan family, and its role as a regulator of various proteins, cells and tissues in the human body makes it an indispensable macromolecule. Heparin-based hydrogels are widely investigated in various applications including implantation, tissue engineering, biosensors, and drug-controlled release due to the 3D-constructs of hydrogels. However, heparin has supply and safety problems because it is usually derived from animal sources, and has the clinical limitations of bleeding and thrombocytopenia. Therefore, analogous heparin-mimicking polymers and hydrogels derived from non-animal and/or totally synthetic sources have been widely studied in recent years. In this review, the progress and potential biomedical applications of heparin-based and heparin-inspired hydrogels are highlighted. We classify the forms of these hydrogels by their size including macro-hydrogels, injectable hydrogels, and nano-hydrogels. Then, we summarize the various fabrication strategies for these hydrogels including chemical covalent bonding, physical conjugation, and the combination of chemical and physical interactions. Covalent bonding includes free radical polymerization of vinyl-containing components, amide bond formation reaction, Michael-type addition reaction, click-chemistry, divinyl sulfone crosslinking, and mussel-inspired coating. Hydrogels physically conjugated via host–guest interaction, electrostatic interaction, hydrogen bonding, and hydrophobic interaction are also discussed. Finally, we conclude with the challenges and future directions for the fabrication and the industrialization of heparin-based and heparin-inspired hydrogels. We believe that this review will attract more attention toward the design of heparin-based and heparin-inspired hydrogels, leading to future advancements in this emerging research field.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...