Polymeric arsenicals as scaffolds for functional and responsive hydrogels†
Abstract
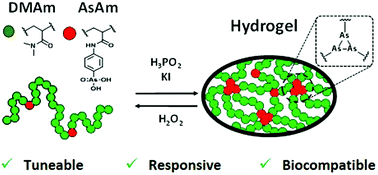
Here arsenohydrogels are introduced for the first time as functional, tuneable and responsive hydrogels. The distinctive redox reactivity of arsenic has been exploited to crosslink high molecular weight (Mw > 300 kDa) polymeric arsenical scaffolds (PDMAmx-co-AsAmy) via reductive coupling of As(V) to As(I) which proceeds with the formation of As–As in the form of As(I)n homocycles. Soft arsenohydrogels (G′ ∼ 400–1700 Pa) that failed in compression tests at low compression and loading are formed when the polymer weight fraction is 2.5 wt%. When the polymer weight fraction is increased to 10 wt% the mechanical properties (stiffness and relaxation) of the arsenohydrogels are significantly improved and correlate with the mole fraction of arsenic (AsAm, y) present in the copolymer scaffolds. Furthermore, increasing the mole fraction of AsAm, reduces the degree of swelling and increases the stability of the gels against hydrolysis and oxidation of the As–As crosslinks. The functionality of the polymeric arsenical scaffolds has also been exploited to load arsenohydrogels with a model organic arsenical drug. The rate and degree of release of the loaded organic arsenical under simulated oxidative stress (H2O2) is inversely proportional to the mole fraction of arsenic in the original polymer scaffold. Finally, the polymeric arsenical scaffolds and the resulting arsenohydrogels have been shown to be non-toxic to NIH/3T3 (mouse fibroblast) and PC3 (human prostate cancer) cell lines. The properties and versitility of the arsenohydrogels alludes to their potential as a functional platform for biomaterials.
- This article is part of the themed collections: Journal of Materials Chemistry B Advisory Board Collection and Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...