Alloying in inverse CeO2/Pd nanoparticles to enhance the electrocatalytic activity for the formate oxidation reaction†
Abstract
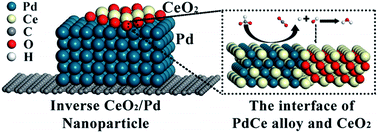
Direct formate fuel cells are promising future power sources due to their environmental friendliness and cost effectiveness. However, their further development and application are hindered by the lack of high-activity catalysts for the formate oxidation reaction (FOR). For the first time, alloying Pd and Ce in inverse CeO2/Pd nanoparticles is observed and confirmed here by a shift of the diffraction peak, an increase of the lattice spacing and a downward shift of the d-band centre. The as-synthesised Pd72Ce28/C catalyst exhibits a mass specific activity of 1.1 A mgPd−1, which is 3.8 times higher than that of the commercial Pd/C catalyst, an improved durability with an activity retention of 11% after 500 cycles and a reproducibility with an activity recovery of 80% after 5 times reactivation. A negative alloy formation energy of −10.44 eV in the Pd3Ce1 alloy is observed from density functional theory calculations, where the effect of alloying Pd and Ce on the FOR catalytic activity can be attributed to the weakened hydrogen binding energy of −1.04 eV and reduced potential limiting barrier of 0.23 eV on the Pd3Ce1 (111) surface. This systematic work, from facile synthesis to theoretical simulations, not only highlights the critical role of the synergy of the electronic effect and oxophilic effect in enhancing the catalytic activity, but also promotes further research on the mechanism of the FOR in alkaline media.


 Please wait while we load your content...
Please wait while we load your content...