Molten salts promoting the “controlled carbonization” of waste polyesters into hierarchically porous carbon for high-performance solar steam evaporation†
Abstract
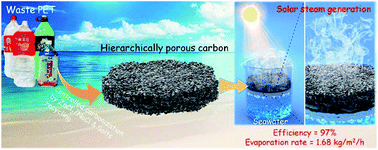
Solar steam generation is emerging as a promising technology to harvest solar energy for diverse applications such as distillation, desalination, and production of freshwater. However, the synthesis of low-cost and high-efficiency photothermal materials remains a challenge for both industrial and academic research. Here, hierarchically porous carbon having an irregular, inter-connected nanoparticle morphology is facilely synthesized through “controlled carbonization” of low-cost waste poly(ethylene terephthalate) (PET) using ZnCl2/NaCl eutectic salts at 550 °C. We prove that ZnCl2 catalyzes the dehydration and decarboxylation of PET to form vinyl-terminated chain fragments and aromatic rings, which subsequently construct a carbon material frame through further cyclization and crosslinking. Meanwhile ZnCl2/NaCl eutectic salts act as porogens to produce mesopores and macropores. The resultant hierarchically porous carbon shows high specific surface area, fast water transportation, high solar absorption efficiency, and low thermal conductivity. Such combined features endow it with a high evaporation rate of 1.68 kg m−2 h−1 under 1 sun irradiation, an energy conversion efficiency of 97%, and a metallic ion removal efficiency of ca. 99.9% for seawater. More importantly, it outperforms the state-of-the-art carbon-based photothermal materials. This work not only reveals the potential of hierarchically porous carbon for application in solar steam generation, but also gives impetus to the sustainable conversion of low-cost waste polyesters into valuable carbon materials by a “controlled carbonization” strategy for energy storage and conversion, environmental remediation, etc.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...