Open Access Article
Open Access ArticleFundamental nanoscale surface strategies for robustly controlling heterogeneous nucleation of calcium carbonate†
Junjie
Zhao
 ab,
Minghui
Wang
ab,
Minghui
Wang
 b,
Mofoluwaso S.
Jebutu
b,
Minghui
Zhu
b and
Karen K.
Gleason
b,
Mofoluwaso S.
Jebutu
b,
Minghui
Zhu
b and
Karen K.
Gleason
 *b
*b
aState Key Laboratory of Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University, 38 Zheda Rd, Hangzhou, China 310027
bDepartment of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave, Cambridge, MA 02139, USA. E-mail: kkg@mit.edu
First published on 4th July 2019
Abstract
Tuning heterogeneous nucleation of CaCO3 could enable new bio-mimic structures and address inorganic fouling in desalination and petroleum industries. However, few fundamental principles exist to guide surface modification for controlling this complex nucleation process. Additionally, industrial applications often require high stability for harsh operational conditions and nanoscale thickness for low heat transfer resistance. Such robust nanoscale coating materials have not yet been reported, motivating the current study of durable ultrathin (≤100 nm) organic covalent networks synthesized by initiated chemical vapor deposition (iCVD). The low surface energy of the iCVD films results in a high energy barrier for CaCO3 heterogeneous nucleation, leading to slow kinetics with extended induction periods. We also found the dominant role of electrochemical oxidation on Cu/Ni surfaces in affecting heterogeneous nucleation of CaCO3 in a hot aqueous environment. With excellent stability and passivation capability, our iCVD coatings effectively inhibit corrosion-induced heterogeneous nucleation, thus reducing the amount of CaCO3 fouling by up to 14 times at 110 °C.
Heterogeneous nucleation of CaCO3 is of fundamental importance for many industrial, geological, and biological processes.1–3 It is widely involved in the formation of biogenic minerals for exoskeletons of many marine species and avian eggshells,1 and in the development of inorganic foulants in desalination and the petroleum industry.4–7 The latter could significantly impair the heat transfer and flow rates in the equipment, and eventually affect the process efficiency and energy consumption.8 Approaches for tuning heterogeneous nucleation of CaCO3 could therefore enable the fabrication of new bio-mimic structures, and provide cost-effective, energy-efficient solutions to inorganic fouling issues in desalination and petroleum industries.
Controlling heterogeneous nucleation of CaCO3 is a non-trivial task because of the complexity of pre-nucleation clusters/precursors and their phase transition paths to polymorphs of CaCO3 that do not follow the classical nucleation theory.1,9–12 In addition, many factors including surface terminal groups, surface energy, roughness, and surface charge and ion interaction can affect heterogeneous nucleation of CaCO3.5 However, it is often not possible to exclusively study one particular factor since adjustment of surface chemistry also leads to changes in other surface properties. Consequently, in spite of the previous attempts to investigate heterogeneous nucleation and growth of CaCO3 on different surfaces,13–21 much is still unknown before a guiding principle can be developed for designing surfaces to control this dynamic process.
Surface modification for tuning heterogeneous nucleation of CaCO3 also requires robustness and thermal stability especially for applications in thermal desalination such as multistage flash (MSF) and multi-effect distillation (MED), and in oil extraction processes. In addition to withstanding wear conditions, these coatings need to be resistant to hydrolysis and erosion when exposing to seawater at temperature as high as 110 °C,22 while maintain a thickness <1 μm to reduce heat transfer resistance.23 A trade-off, however, is often present between the low film thickness desired to maintain thermal conduction and the stability of the coating layer. In most cases, film thickness was increased to achieve stability and robustness of the surface at the expense of reducing thermal conduction across the layer.7,24,25 So far, nanoscale robust coatings stable at extreme operational conditions for controlling heterogeneous nucleation of CaCO3 have not yet been reported.
Here we report a novel method to control heterogeneous nucleation of CaCO3 using nanoscale polymer coatings deposited via initiated chemical vapor deposition (iCVD). iCVD is advantageous in controlling film thickness and obtaining conformal coatings, which is crucial for applications in desalination and petroleum equipment. In order to avoid significant thermal insulation, the organic films applied to the metal heat exchanger surfaces must be ultrathin (<1 μm).23 Due to the absence of surface tension, and hence dewetting effects, iCVD is ideal for forming such ultrathin pinhole-free organic films. Additionally, the metal surfaces in heat transfer equipment typically have significant roughness (on the orders of tens of nm or higher). To equally cover all the features in these surfaces requires a conformal coating method, such as iCVD.26
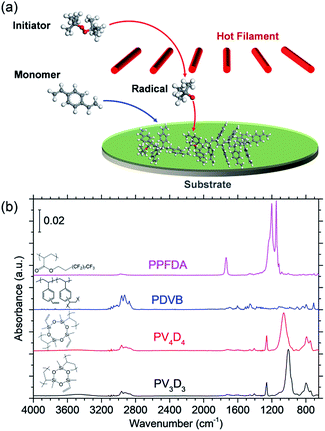
We carefully selected four iCVD polymers from over 70 candidates available for iCVD chemistry based on their surface energy, film roughness and durability.26 Polydivinylbenzene (PDVB), Poly(1,3,5-trivinyl-1,3,5-trimethylcyclotrisiloxane) (PV3D3), Poly(1,3,5,7-tetra-vinyl-1,3,5,7-tetramethylcyclotetra-siloxane) (PV4D4) and Poly(1H,1H,2H,2H-perfluorodecyl acrylate) (PPFDA) were synthesized using iCVD processes similar to previous reports (experimental details shown in ESI†).27–30 In a typical iCVD process, free radicals are generated from tert-butyl peroxide (TBPO) vapors flowing past an array of hot filaments. These radicals can further initiate the polymerization of the monomers adsorbed on the growth surface (Fig. 1a).
 | ||
| Fig. 1 (a) Schematic illustration for initiated chemical vapor deposition (iCVD). (b) FTIR spectra for iCVD PV3D3, PV4D4, PDVB and PPFDA. | ||
Fig. 1b shows the Fourier transform infrared (FTIR) spectra for iCVD PV3D3, PV4D4, PDVB and PPFDA. These spectra are consistent with prior reports, confirming the successful synthesis of the targeted polymer structures.27,29–31 For the polymers synthesized from multi-vinyl monomers, only low absorbance was found for the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 1597 cm−1 for PV3D3, at 1600 cm−1 for PV4D4, and at 1630 cm−1 for PDVB, respectively,27,29,31 indicating negligible pendant vinyl groups in these polymers after iCVD. For iCVD PPFDA, complete loss of the ν(C
C) at 1597 cm−1 for PV3D3, at 1600 cm−1 for PV4D4, and at 1630 cm−1 for PDVB, respectively,27,29,31 indicating negligible pendant vinyl groups in these polymers after iCVD. For iCVD PPFDA, complete loss of the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) at 1639 cm−1 in the monomer was achieved after polymerization,32 revealing full conversion of the double bonds.
C) at 1639 cm−1 in the monomer was achieved after polymerization,32 revealing full conversion of the double bonds.
We deposited these iCVD polymer films on Cu/Ni alloy foils that have similar composition with the tube materials used in the heat exchangers in MSF plants.33 Film thickness was controlled at ca. 100 nm based on in situ interferometry during the iCVD processes. This thickness was chosen because it ensured the formation of continuous, pinhole-free films for good passivation while maintaining small heat transfer resistance on the Cu/Ni surface.23 Grazing incidence X-ray diffraction (GIXRD) for the iCVD-coated Cu/Ni foils is shown in Fig. S1.† The diffraction peaks shown at low 2θ angles for PPFDA represent the lamellar structure of this polymer,34 while the other iCVD polymer films are amorphous. Atomic force microscopy (AFM) reveals that pristine Cu/Ni alloy foil has aligned surface features with a roughness of 37.8 ± 11.0 nm (Fig. S2†). The intrinsic roughness of the iCVD polymer films measured on Si substrates is generally less than 0.5 nm, except for iCVD PPFDA (15.8 ± 1.5 nm) due to its crystalline structure (Fig. S3†). The roughness of iCVD coated Cu/Ni foils is quite similar to the uncoated substrate (Table S1†), confirming the combination of the conformal coverage and the low intrinsic roughness of the iCVD surface modification layer.
The surface energy of the iCVD polymers was determined using contact angle measurements with multiple liquids with known surface tension, and compared with common commercial polymers35,36 (Fig. S4†). Polytetrafluoroethylene (PTFE) and polydimethylsiloxane (PDMS) represent the lower bound of surface energy (ca. 20 mN m−1) among common polymer materials. The surface energy of our cyclosiloxane-based iCVD PV3D3 (21.89 ± 2.91 mN m−1) and PV4D4 (19.96 ± 2.82 mN m−1) is quite comparable with that of PTFE and PDMS, while the surface energy of iCVD PPFDA (9.36 ± 1.32 mN m−1) was found at least 50% smaller than PTFE and PDMS.
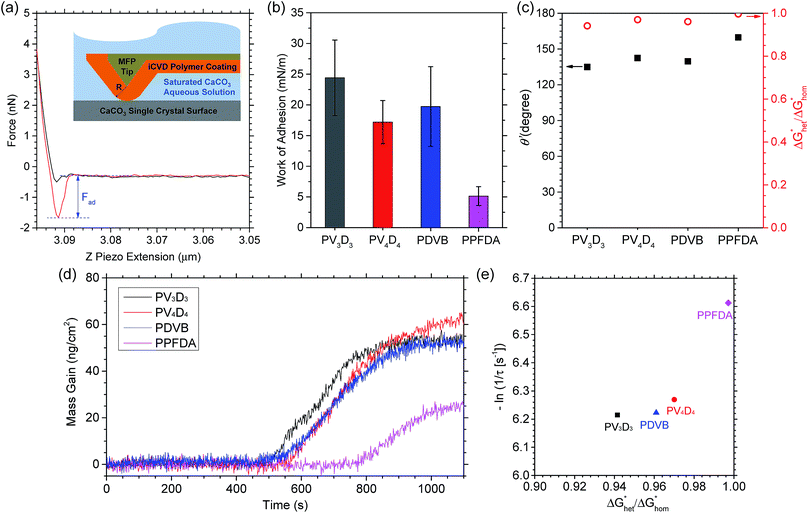
We investigated the adhesion between CaCO3 and the iCVD polymer films using molecular force probe (MFP). iCVD-coated MFP cantilevers were employed to probe the surface of a CaCO3 single crystal (dimension: 10 mm × 10 mm × 0.5 mm) in an aqueous solution of saturated CaCO3 (Fig. 2a). Force response was recorded when the MFP probe was approaching and retracting from the CaCO3 surface. Work of adhesion (Wad) was calculated from the measured adhesion forces using Johnson–Kendall–Roberts (JKR) model (details shown in the ESI†).37 As shown in Fig. 2b, iCVD PPFDA exhibits ultra-low Wad (5.14 ± 1.54 mN m−1), indicating a non-sticking surface for CaCO3. This surface property is highly desired for desalination and petroleum equipment, as it could prevent the CaCO3 precipitated in solution from adhering to the substrate surface. Compared with untreated Cu, SiO2 coated Cu, and Cu coupled with triethoxyvinylsilane,38 the Wad between CaCO3 and all the four iCVD polymers are significantly smaller (Table S2†). The Wad for CaCO3 on iCVD PPFDA, PV4D4 and PV3D3 increases consistently with the surface energy of the corresponding polymer. Although iCVD PDVB exhibits higher surface energy than iCVD PV3D3, the Wad for CaCO3 on iCVD PDVB was found lower than that on iCVD PV3D3. This is possibly because the surface polarity of iCVD PDVB is significantly lower than PV3D3 (Table S3†). Polar interactions originate from the surface charge of CaCO3 (ref. 39) and the induced orientation of molecular dipoles in polymers.40 Large opposite polarity leads to strong adhesion, resulting in high work of adhesion.41
With the analyzed Wad and the reported interfacial energy between water and CaCO3 (γLS = 83 mN m−1),42 we estimated the contact angle (θ′) of CaCO3 on the iCVD polymers using Young–Dupré equation (Fig. 2c).
Wad = γLS(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ′) θ′) | (1) |
We found the θ′ is higher than 130° for all the four iCVD polymers (Fig. 2c), indicating that these iCVD surfaces are not favored for CaCO3. Assuming that the heterogeneous nucleation of CaCO3 follows the classical nucleation theory, we further estimated the energy barrier as a ratio of the change of free energy during CaCO3 heterogeneous nucleation to that during CaCO3 homogeneous nucleation (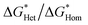 ). Here we use a simplified model with a spherical nuclei cap formed on a flat mold (Scheme S1†) to describe heterogeneous nucleation of CaCO3 on iCVD polymer surfaces. The energy barrier for heterogeneous nucleation (
). Here we use a simplified model with a spherical nuclei cap formed on a flat mold (Scheme S1†) to describe heterogeneous nucleation of CaCO3 on iCVD polymer surfaces. The energy barrier for heterogeneous nucleation (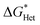 ) is given by
) is given by
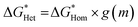 | (2) |
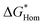 is the energy barrier for homogenous nucleation, the geometric factor g(m) is defined as a function of the contact angle (m = cos
is the energy barrier for homogenous nucleation, the geometric factor g(m) is defined as a function of the contact angle (m = cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ′)
θ′)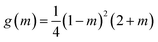 | (3) |
As shown in Fig. 2c, 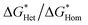 is over 90% for all the tested iCVD surfaces, indicating that these iCVD polymer coatings could effectively inhibit heterogeneous nucleation of CaCO3.
is over 90% for all the tested iCVD surfaces, indicating that these iCVD polymer coatings could effectively inhibit heterogeneous nucleation of CaCO3.
Fig. 2d shows the mass gain measured via quartz crystal microbalance (QCM) during the nucleation and growth of CaCO3 in a flow cell. A delay of nucleation was observed for all the four iCVD-coated QCM surfaces. iCVD PPFDA exhibits the longest induction period among the iCVD polymers, consistent with the highest nucleation energy found from adhesion analysis. Since induction time (τ) is inversely proportional to nucleation rate,43 we found that the nucleation rate of CaCO3 decreases with the increase of CaCO3 contact angle on the iCVD polymers. τ can also be directly correlated with 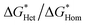 through eqn (4) (derivation shown in ESI†)
through eqn (4) (derivation shown in ESI†)
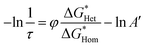 | (4) |
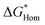 to the product of Boltzmann constant and temperature (kBT). Both A′ and φ are constant for CaCO3 nucleation at given temperature. As shown in Fig. 2e, −ln(1/τ) increases with
to the product of Boltzmann constant and temperature (kBT). Both A′ and φ are constant for CaCO3 nucleation at given temperature. As shown in Fig. 2e, −ln(1/τ) increases with 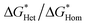 , confirming that the nanoscale surface modification using iCVD enables the control of CaCO3 nucleation kinetics.
, confirming that the nanoscale surface modification using iCVD enables the control of CaCO3 nucleation kinetics.
In order to demonstrate the potential of the nanoscale iCVD coatings for controlling CaCO3 nucleation in thermal desalination, we further evaluated these iCVD polymer films in conditions similar to MSF processes. Uncoated and iCVD coated Cu/Ni foils were immersed in a mixed aqueous solution of 10 mmol L−1 CaCl2 and 2 mmol L−1 NaHCO3, which mimics the concentration of Ca2+ and HCO3− typically found in sea water. The pressure of the distillation apparatus was tuned to obtain different boiling points of the synthetic seawater (50–110 °C), similar to the typical conditions in the external brine heaters and the heat exchangers in an MSF plant.5
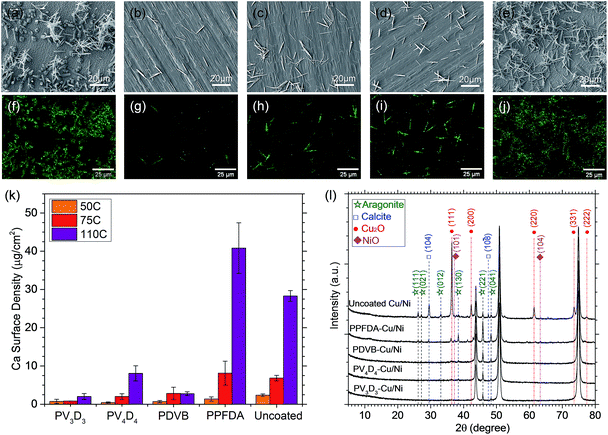
Fig. 3a–j compare the surface of the Cu/Ni foils after the CaCO3 fouling test at 110 °C. Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) mapping show large amount of CaCO3 particles with different morphology on the uncoated Cu/Ni (Fig. 3a). The small particles (average size = 544 ± 170 nm) formed densely on the substrate surface (Fig. S5†) were found to be the main corrosion product of Cu2O via GIXRD (Fig. 3l). The CaCO3 crystals observed on top of the corrosion products were identified as aragonite and calcite. This is consistent with previous reports of inorganic foulant layers on top of corrosion products in MSF,33,44 and suggests that the corrosion products act as nucleation sites for CaCO3. The amounts of the CaCO3 were further quantitatively analyzed by digesting the foulant in dilute nitric acid and using inductively-coupled plasma optical emission spectroscopy (ICP-OES). Fig. 3k shows that the Ca surface density on uncoated Cu/Ni is 2.38 ± 0.34 μg cm−2, 6.84 ± 0.72 μg cm−2 and 28.3 ± 1.4 μg cm−2 after CaCO3 fouling tests at 50 °C, 75 °C and 110 °C, respectively.
In contrast to uncoated Cu/Ni, Cu/Ni coated with iCVD PV3D3, PV4D4 and PDVB show significantly reduced amounts of CaCO3 crystals after the fouling test at 110 °C (Fig. 3b–d and g–i). ICP-OES results reveal that iCVD PV3D3 coated Cu/Ni enables almost 14 times lower CaCO3 fouling than uncoated Cu/Ni at 110 °C. For the CaCO3 fouling test at 75 °C, the Ca surface density on Cu/Ni decreases by over 8 times with the protection of iCVD PV3D3 coatings. This significant reduction of CaCO3 formation in the temperature range for the brine heaters and the first few MSF stages where scaling is most likely to happen suggests that longer operational periods with fewer shutdowns for maintenance could be achieved for future MSF plants. For the CaCO3 fouling test at 50 °C, the Ca surface density is all below 0.8 μg cm−2 for Cu/Ni coated with iCVD PV3D3, PV4D4 and PDVB, at least 3 times lower than uncoated Cu/Ni surfaces. These results confirm that our iCVD polymer coatings could be applied to inhibit CaCO3 fouling in MSF plants for the entire operational temperature range.
Compared with uncoated Cu/Ni surface where both calcite and aragonite were observed, aragonite appears to be the dominant phase on iCVD coated Cu/Ni. This can be explained by the known phase transition from aragonite to calcite in hot aqueous fluid,45 as calcite is more thermodynamically stable than aragonite.1 The calcite observed on uncoated Cu/Ni was possibly converted from the aragonite, while significant nucleation delay on iCVD coated Cu/Ni resulted in aragonite as the dominant phase.
We noticed that iCVD PPFDA coated Cu/Ni shows promoted heterogeneous nucleation of CaCO3 for the fouling tests at 75 °C and 110 °C compared with uncoated Cu/Ni. This is because this acrylate polymer is prone to hydrolysis in hot aqueous environment. Optical microscopy reveals the erosion of iCVD PPFDA films during the exposure to boiling water. Consequently, the loss of passivation leads to the corrosion of Cu/Ni, as confirmed by GIXRD (Fig. 3l). The degraded polymer chains in the vicinity of the surface and the corrosion products of Cu/Ni are likely to promote the heterogeneous nucleation of CaCO3. In contrary, no corrosion products were found on Cu/Ni coated with iCVD PV3D3, PV4D4 and PDVB, indicating that these cross-linked iCVD polymer films have excellent stability and allow good passivation for Cu/Ni.
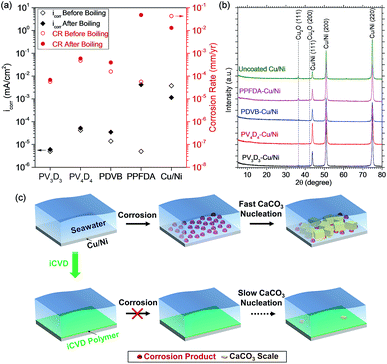
In order to better understand the role of surface corrosion in promoting the heterogeneous nucleation of CaCO3, We applied electrochemical methods to investigate the corrosion rates for uncoated and iCVD coated Cu/Ni foils (film thickness = ca. 100 nm) before and after exposure to boiling water at 100 °C for 2 h. Fig. 4a shows the corrosion current density (icorr) extracted from linear sweep voltammetry (LSV) via Tafel analysis and the corresponding corrosion rates. Compared with uncoated Cu/Ni, the icorr of iCVD-coated Cu/Ni foils prior to the exposure to boiling water is 2–3 orders of magnitude smaller. Notably, as-deposited iCVD PPFDA shows extremely low icorr (5.0 × 10−6 mA cm−2). This excellent passivation performance prior to the exposure to hot aqueous environment also explains the abovementioned slow nucleation kinetics of CaCO3 on iCVD PPFDA at room temperature. After exposure to boiling water for 2 h, however, the icorr for iCVD PPFDA increases dramatically from 5.0 × 10−6 mA cm−2 to 4.2 × 10−3 mA cm−2. GIXRD also shows Cu2O corrosion product on PPFDA coated Cu/Ni (Fig. 4b). These results again confirm the loss of passivation for Cu/Ni due to the erosion of iCVD PPFDA.
In addition, we found that the trend of CaCO3 nucleation and growth on iCVD-coated Cu/Ni surfaces at 110 °C agrees well with the corresponding corrosion rate measured after exposure to boiling water. iCVD PV3D3 coated Cu/Ni exhibits the lowest corrosion rate (6.8 × 10−5 mm per year) among the four iCVD coatings, which is 191 times slower than uncoated Cu/Ni. The ultra-low corrosion rate explains the lowest amount of CaCO3 formed on iCVD PV3D3 coated Cu/Ni at 110 °C, although the nucleation energy barrier and the induction time at room temperature for iCVD PV3D3 were found lower than other iCVD polymers. These results clearly indicate the dominant role of surface electrochemical oxidation of Cu/Ni in the heterogeneous nucleation of CaCO3. The mechanism is proposed in Fig. 4c for the corrosion-induced heterogeneous nucleation of CaCO3 on Cu/Ni in hot aqueous conditions and the inhibition strategy using nanoscale iCVD coatings.
Finally, we evaluated the robustness of our iCVD anti-scaling coatings in high-temperature fouling tests for extended hours. After exposure to the synthetic seawater at 110 °C for 72 h, severe corrosion was found on uncoated Cu/Ni foils (Fig. S7b†). Consequently, the Ca surface density on uncoated Cu/Ni is as high as 39.6 μg cm−2, indicating large amount of CaCO3 foulant (Fig. S7a†). In contrast, the Ca surface density is generally less than 10 μg cm−2 for Cu/Ni coated with iCVD PV3D3, PV4D4 and PDVB. Especially, the suppression of CaCO3 fouling is maintained over 10 times under the protection with iCVD PV3D3, demonstrating the excellent stability of nanoscale iCVD coatings for long-term control of CaCO3 nucleation in thermal desalination.
Conclusions
In summary, we have developed a series of robust nanoscale iCVD polymer coatings for controlling heterogeneous nucleation of CaCO3 in various conditions. Polymer coating materials with low surface energy and polarity were found with low adhesion to CaCO3 and high nucleation barrier for heterogeneous nucleation of CaCO3. In addition, for practical conditions in thermal desalination, the chemical stability and the surface passivation ability to inhibit surface corrosion are also important factors for designing anti-fouling coatings to control CaCO3 surface nucleation. Crosslinked covalent organic networks like iCVD PV3D3 have been synthesized as robust nanoscale coatings that exhibit both ultra-low corrosion rate of Cu/Ni and excellent performance for controlling CaCO3 nucleation in hot aqueous environment. The iCVD surface modification reported here represents a promising strategy to control CaCO3 fouling in thermal desalination and petroleum industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Kuwait Foundation for the Advancement of Sciences (KFAS) (P31475EC01) for funding support through the Kuwait-MIT Center for Natural Resources and the Environment at MIT. JZ acknowledges the startup funding from Zhejiang University and the funding supported by State Key Laboratory of Chemical Engineering.References
- L. B. Gower, Chem. Rev., 2008, 108, 4551 CrossRef CAS PubMed
.
- N. A. J. M. Sommerdijk and G. de With, Chem. Rev., 2008, 108, 4499 CrossRef CAS PubMed
.
- A. V. Radha and A. Navrotsky, Rev. Mineral. Geochem., 2013, 77, 73 CrossRef CAS
.
- D. M. Warsinger, J. Swaminathan, E. Guillen-Burrieza, H. A. Arafat and J. H. Lienhard V, Desalination, 2015, 356, 294 CrossRef CAS
.
- J. Zhao, M. Wang, H. M. S. Lababidi, H. Al-Adwani and K. K. Gleason, Desalination, 2018, 442, 75 CrossRef CAS
.
- J. Li, M. Tang, Z. Ye, L. Chen and Y. Zhou, J. Dispersion Sci. Technol., 2017, 38, 661 CrossRef CAS
.
- M. M. Vazirian, T. V. J. Charpentier, M. de Oliveira Penna and A. Neville, J. Pet. Sci. Eng., 2016, 137, 22 CrossRef CAS
.
-
F. Rahman and Z. Amjad, in Sci. Technol. Ind. Water Treat., CRC Press, 2010, pp. 271–396 Search PubMed
.
- D. Gebauer, A. Völkel and H. Cölfen, Science, 2008, 322, 1819 CrossRef CAS PubMed
.
- D. Gebauer, P. N. Gunawidjaja, J. Y. P. Ko, Z. Bacsik, B. Aziz, L. Liu, Y. Hu, L. Bergström, C.-W. Tai, T.-K. Sham, M. Edén and N. Hedin, Angew. Chem., Int. Ed., 2010, 49, 8889 CrossRef CAS PubMed
.
- M. H. Nielsen, S. Aloni and J. J. D. Yoreo, Science, 2014, 345, 1158 CrossRef CAS PubMed
.
- R. Jens, K. Matthias and N. Luc, Angew. Chem., Int. Ed., 2014, 53, 12380 Search PubMed
.
- D. M. Duffy and J. H. Harding, Langmuir, 2004, 20, 7630 CrossRef CAS PubMed
.
- S. Yamanaka, N. Ito, A. Shimosaka, Y. Shirakawa and J. Hidaka, Cryst. Growth Des., 2009, 9, 3245 CrossRef CAS
.
- Q. Hu, M. H. Nielsen, C. L. Freeman, L. M. Hamm, J. Tao, J. R. I. Lee, T. Y. J. Han, U. Becker, J. H. Harding, P. M. Dove and J. J. D. Yoreo, Faraday Discuss., 2013, 159, 509 RSC
.
- A. Fernandez-Martinez, Y. Hu, B. Lee, Y.-S. Jun and G. A. Waychunas, Environ. Sci. Technol., 2013, 47, 102 CrossRef CAS PubMed
.
- L. M. Hamm, A. J. Giuffre, N. Han, J. Tao, D. Wang, J. J. D. Yoreo and P. M. Dove, Proc. Natl. Acad. Sci. U.S.A., 2014, 111, 1304 CrossRef CAS PubMed
.
- Q. Li, A. Fernandez-Martinez, B. Lee, G. A. Waychunas and Y.-S. Jun, Environ. Sci. Technol., 2014, 48, 5745 CrossRef CAS PubMed
.
- N. R. Chevalier, J. Phys. Chem. C, 2014, 118, 17600 CrossRef CAS
.
- P. J. M. Smeets, K. R. Cho, R. G. E. Kempen, N. A. J. M. Sommerdijk and J. J. De Yoreo, Nat. Mater., 2015, 14, 394 CrossRef CAS PubMed
.
- Y.-S. Jun, D. Kim and C. W. Neil, Acc. Chem. Res., 2016, 49, 1681 CrossRef CAS PubMed
.
- A. Al-Karaghouli and L. L. Kazmerski, Renew. Sustain. Energy Rev., 2013, 24, 343 CrossRef CAS
.
- A. T. Paxson, J. L. Yagüe, K. K. Gleason and K. K. Varanasi, Adv. Mater., 2014, 26, 418 CrossRef CAS PubMed
.
- W. C. Cheong, P. H. Gaskell and A. Neville, J. Cryst. Growth, 2013, 363, 7 CrossRef CAS
.
- T. V. Charpentier, A. Neville, S. Baudin, M. J. Smith, M. Euvrard, A. Bell, C. Wang and R. Barker, J. Colloid Interface Sci., 2015, 444, 81 CrossRef CAS PubMed
.
- A. M. Coclite, R. M. Howden, D. C. Borrelli, C. D. Petruczok, R. Yang, J. L. Yagüe, A. Ugur, N. Chen, S. Lee, W. J. Jo, A. Liu, X. Wang and K. K. Gleason, Adv. Mater., 2013, 25, 5392 CrossRef CAS PubMed
.
- J. Zhao, M. Wang and K. K. Gleason, Adv. Mater. Interfaces, 2017, 4, 1700270 CrossRef
.
- H. Moon, H. Seong, W. C. Shin, W.-T. Park, M. Kim, S. Lee, J. H. Bong, Y.-Y. Noh, B. J. Cho, S. Yoo and S. G. Im, Nat. Mater., 2015, 14, 628 CrossRef CAS PubMed
.
- N. J. Trujillo, Q. Wu and K. K. Gleason, Adv. Funct. Mater., 2010, 20, 607 CrossRef CAS
.
- S. Kim, H. Sojoudi, H. Zhao, D. Mariappan, G. H. McKinley, K. K. Gleason and A. J. Hart, Sci. Adv., 2016, 2, e1601660 CrossRef PubMed
.
- W. S. O'Shaughnessy, M. Gao and K. K. Gleason, Langmuir, 2006, 22, 7021 CrossRef PubMed
.
- M. Gupta and K. K. Gleason, Langmuir, 2006, 22, 10047 CrossRef CAS PubMed
.
- A. S. El Din, M. E. El-Dahshan and R. A. Mohammed, Desalination, 2005, 177, 241 CrossRef CAS
.
- A. Perrotta, P. Christian, A. O. F. Jones, F. Muralter and A. M. Coclite, Macromolecules, 2018, 51, 5694 CrossRef CAS PubMed
.
-
J. C. Arnold, in Compr. Struct. Integr., ed. I. Milne, R.O. Ritchie and B. Karihaloo, Pergamon, Oxford, 2003, pp. 281–319 Search PubMed
.
-
G. L. Jialanella, in Adv. Struct. Adhes. Bond., ed. D.A. Dillard, Woodhead Publishing, 2010, pp. 237–264 Search PubMed
.
- E. Barthel, J. Phys. D: Appl. Phys., 2008, 41, 163001 CrossRef
.
- R. Hidema, T. Toyoda, H. Suzuki, Y. Komoda and Y. Shibata, Int. J. Heat Mass Transf., 2016, 92, 603 CrossRef CAS
.
- S. S. Lee, F. Heberling, N. C. Sturchio, P. J. Eng and P. Fenter, J. Phys. Chem. C, 2016, 120, 15216–15223 CrossRef CAS
.
- A. J. Kinloch, J. Mater. Sci., 1980, 15, 2141–2166 CrossRef CAS
.
-
D. M. Mattox, in Handb. Phys. Vap. Depos. PVD Process, ed. D. M. Mattox, William Andrew Publishing, 2nd edn, 2010, pp. 439–474 Search PubMed
.
- O. Söhnel and J. W. Mullin, J. Cryst. Growth, 1978, 44, 377 CrossRef
.
- W. Wu and G. H. Nancollas, Adv. Colloid Interface Sci., 1999, 79, 229 CrossRef CAS PubMed
.
- A. G. I. Dalvi, N. M. Kither Mohammad, S. Al-Sulami, K. Sahul and R. Al-Rasheed, Desalination, 2000, 129, 173 CrossRef CAS
.
- M. E. Berndt and W. E. Seyfried, Geochim. Cosmochim. Acta, 1999, 63, 373–381 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta04341a |
| This journal is © The Royal Society of Chemistry 2019 |