Pt-like hydrogen evolution on a V2O5/Ni(OH)2 electrocatalyst†
Abstract
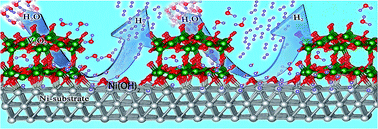
We report a highly efficient and cost-effective binder-free catalyst for the hydrogen evolution reaction (HER) using V2O5 particles on nickel foam (NF) (V2O5/Ni(OH)2@NF). This low-cost catalyst exhibits Pt-like activity with a low overpotential of 39 mV at 10 mA cm−2 (lowest among V-based materials which are known to be generally non-explosive and safe) and long-term stability in a 1 M KOH solution. The overall performance is highly comparable to that of a commercial 20% Pt/C catalyst on NF. Furthermore, the V2O5/Ni(OH)2@NF outperforms the Pt/C catalyst at a higher current density (100 mA cm−2) which is more preferable for industrial applications. First principles calculations show that the remarkable HER activity is ascribed to the near-zero adsorption free energy (ΔGH*) on the Ni-site of Ni(OH)2@NF and the Ni- and O-sites of in situ generated V2O5@NF, due to the charge transfer arising from adsorbed O atoms on Ni(111), along with high conductivity of NF. O-adsorption on the Ni transition metal surface downshifts the d-band center of the transition metal, which helps in quick hydrogen desorption by weakening the hydrogen binding strength. As a result, most Ni fcc sites of V2O5/Ni(OH)2@NF are more active than pristine Ni fcc sites. The V2O5/Ni(OH)2@NF catalyst initiates overall water splitting at 1.53 V in a 6 M KOH solution for solar-to-hydrogen generation in a two-electrode set-up using a solar panel.


 Please wait while we load your content...
Please wait while we load your content...