Highly efficient self-healable and dual responsive hydrogel-based deformable triboelectric nanogenerators for wearable electronics†
Qingbao
Guan
 a,
Guanghui
Lin
b,
Yuzhu
Gong
c,
Jingfeng
Wang
b,
Weiyi
Tan
c,
Dequan
Bao
d,
Yina
Liu
e,
Zhengwei
You
a,
Guanghui
Lin
b,
Yuzhu
Gong
c,
Jingfeng
Wang
b,
Weiyi
Tan
c,
Dequan
Bao
d,
Yina
Liu
e,
Zhengwei
You
 a,
Xuhui
Sun
a,
Xuhui
Sun
 d,
Zhen
Wen
d,
Zhen
Wen
 *df and
Yue
Pan
*df and
Yue
Pan
 *b
*b
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, International Joint Laboratory for Advanced Fiber and Low-dimension Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, China
bGuangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Department of Cardiology, Medical Research Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, China. E-mail: panyue@mail.sysu.edu.cn
cState and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China
dInstitute of Functional Nano and Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials and Devices, Soochow University, Suzhou 215123, China. E-mail: wenzhen2011@suda.edu.cn
eDepartment of Mathematical Sciences, Xi'an Jiaotong-Liverpool University, Suzhou 215123, China
fNantong Textile & Silk Industrial Technology Research Institute, Jiangsu Industrial Technology Research Institute of Textile & Silk, Nantong 226314, China
First published on 8th April 2019
Abstract
Self-healable soft conductors, which can withstand certain degrees of deformation and can recover from damage spontaneously, are essential for wearable applications. In this work, a soft hydrogel based self-healing triboelectric nanogenerator (HS-TENG), which is highly deformable, and both mechanically and electrically self-healable, has been successfully fabricated from a poly(vinyl alcohol)/agarose hydrogel. The incorporation of photothermally active polydopamine particles and multiwalled carbon nanotubes (MWCNTs) allows the HS-TENG to be physically self-healed in ∼1 min upon exposure to near-infrared (NIR) light. At the same time, the chemical self-healing of the HS-TENG can be triggered by water spraying at 25 °C when introducing water-active dynamic borate bonds into the hydrogel. The applicability of the HS-TENG as a soft energy device to harvest human motion energies has been demonstrated. By tapping the HS-TENG with various deformations, the rectified electricity can charge commercial LEDs with sustainable energy. Working in single-electrode mode, the electrical outputs of the HS-TENG in terms of short-circuit transferred charge (Qsc), open circuit voltage (Voc) and short-circuit current (Isc) reach ∼32 nC, ∼95 V and ∼1.5 μA, respectively, and remain stable even with 200% strain since the MWCNTs disperse evenly in the matrix and play the role of conductive fillers in the HS-TENG.
Introduction
As the tempo of modern life continues to increase, wearable devices are attracting extensive attention due to their portable nature, and the seamless and convenient access to electronic devices,1,2 saving people a large amount of time by allowing the wearers to obtain information in real time. Nevertheless, one subsequent challenge is to provide accordingly soft power sources to drive these devices. In the design of soft power sources for wearable devices the following features should be considered.3 First, for the sake of achieving great adhesion of the devices to the body or tissues, the property of being bendable is necessary.4 Second, the materials should be non-toxic, which would be an indispensable prerequisite to be biocompatible enough to be applied in wearable devices.5,6 Third, to make them more credible and to achieve a satisfactory performance of wearable devices, the power source should be sustainable to lower the potential risks brought about by replacing it from the inside of wearable devices.7,8 A flexible, innoxious and reliable material is desirable for producing power sources of wearable devices.Based on the combination of the triboelectric effect and electrostatic induction, triboelectric nanogenerators (TENGs) have been achieving great progress as novel power sources.9–12 In this way, a feasible strategy to convert the energy produced by mechanical motions into electrical signals can be realized using TENGs, containing intrinsic traits such as diminutive size, high integration and low weight, which make them sustainable powers source applied in wearable electronics.13–15 However, TENGs could potentially get damaged because of unintentional destructive motions, such as external mechanical stimuli or internal mechanical frictions, which would affect the performance of TENGs.16,17 Hence, designing a self-healable TENG to elongate the life span is urgent.
With respect to the wearable applications of TENGs, the flexibility and toxicity must be considered thoroughly. The hydrogel, one kind of naturally existing environmentally friendly material containing a cross-linking network of polymer chains, fits the above-mentioned features to be utilized in TENGs.18–22 Poly(vinyl alcohol) (PVA) based hydrogels with outstanding biocompatibility and excellent mechanical properties could be used as the matrix of TENGs for wearable devices.23 In order to be utilized in wearable devices, some modifications should be made to make TENGs self-healable to ensure reliability and sustainability.24–29 Particularly, it is worth fabricating a novel TENG with a self-healable electrode layer, which is different from previous research studies focusing on the self-healable triboelectric layer. In particular, their self-healing processes take place at high temperature (∼90 °C) for hours (Table S1†).17,25 A cracked triboelectric layer does not significantly influence the final electric performance since the output of a triboelectric layer is determined by total area, no matter whether the layer is intact or broken; however, the electrical output performance of TENGs decreases dramatically when the electrode layer is damaged or broken,12 and hence, a self-healable electrode layer is essential and meaningful.
Herein, we present a novel design of an environmentally friendly hydrogel based self-healing TENG (HS-TENG) achieved by introducing photothermally active polydopamine particles (PDAPs) and multiwalled carbon nanotubes (MWCNTs), and water-active dynamic borate bonds into a PVA/agarose hydrogel. The resultant HS-TENG can be highly efficiently self-healed under both the exposure to near-infrared (NIR) light in ∼1 min and water at 25 °C in 5 min according to physical (melting/recrystallization of hydrogel microcrystallites) and chemical (reversible covalent crosslinking of borate bonds) healing mechanisms, respectively. The HS-TENG achieves high stretchability, and mechanical and electrical healing efficiency, respectively. The applicability of the HS-TENG as a soft energy device to harvest human motion energies has also been demonstrated by tapping the HS-TENG with various deformations. This work promisingly extends the reliability of TENGs for use in wearable devices and provides dual mode self-healing under different conditions.
Results and discussion
To fabricate an environmentally friendly, self-healable and stretchable TENG applied in wearable devices, a PVA/agarose-based hydrogel was chosen as the matrix, as shown in Fig. 1. PDAPs were incorporated to improve the mechanical strength since PDAPs contain various functional groups, such as amines and catechol, which will form reversible hydrogen bonds between the hydroxyl groups of PVA crystallites and PDAPs.30 PDAPs were prepared from a weak alkaline aqueous solution through the oxidative polymerization of dopamine. The dopamine molecules were spontaneously oxidized during the polymerization process, forming spherical PDAPs via intra/inter-molecular cross-linking (Fig. 1a).31 The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images show that the obtained PDAPs are spherical with a uniform size distribution, and the average particle size is ∼220 nm (Fig. 1b and S1†). Besides the reinforcement on mechanical strength, PDAPs play another important role as a photothermal conversion agent to trigger self-healing upon exposure to NIR laser light (808 nm). The temperature of the PDAPs/water solution can be tuned by adjusting the PDAP content and NIR light intensity. At a NIR power intensity of 2 W cm−2, a medium amount of PDAPs (600 μg mL−1) can increase the temperature of the PDAPs/water solution up to 60 °C in 6 min, and shows the highest efficiency (heat/filler ratio) compared to other concentrations (Fig. 1c). Therefore, the PVA/PDAP hydrogel was fabricated with a PDAP concentration of 600 μg mL−1. It was found that PDAPs have a notable photothermal effect on the PVA/PDAP hydrogel, the temperature of which can reach 120 °C in ∼1 min (Fig. 1d). This mild and efficient local temperature control provides a solid foundation for the functionalization and application of the PVA/PDAP hydrogel. In addition, the borate bonds consisting of borate ions complexed to the hydroxyl groups of PVA crystallites provide dynamic covalent crosslinking borate bonds via absorbing water. Considering the good conductivity of MWCNTs,32–34 they were chosen as conductive fillers doped in the matrix. Eventually, a highly stretchable PVA/PDAP/MWCNT hydrogel (stretch ratio λ ∼ 4) is obtained (Fig. 1e). Compared with the pure PVA hydrogel (Fig. S2†), PDAPs can be evenly dispersed in the composite hydrogel without obvious aggregation. With the introduction of MWCNTs, the resultant hydrogels show a rougher but homogeneous morphology, indicating that the nanofillers disperse evenly in the matrix.To visually demonstrate the healing event, a PVA/PDAP/MWCNT hydrogel was cut into two pieces and they attached back upon water spraying and NIR irradiation, as displayed in Fig. 2. It can be seen that the broken samples are healed thoroughly by both water and NIR self-healing processes, and the healed sample could retain satisfactory stretchability (Fig. 2a and b, Movies S1 and S2†). For the water triggered self-healing mechanism, owing to the fact that covalent crosslinking borate bonds are dynamic and reversible with the assistance of existing water, the borate ions complexed to the hydroxyl groups of PVA crystallites allow a chemical self-healing process to take place (Fig. 2c). In the case of the NIR remotely induced healing process, the small divergence of NIR ensures facile handling of the radiation beam,35 enabling its precise confinement in targeted cracks and minimizing unnecessary influences on other parts. As the PVA hydrogel crystallites’ reversible melting proceeds, constant reshuffling of the molecular chains leads to recrystallization. This suggests that a physical self-healing process under NIR irradiation can take place in the PVA/PDAP/MWCNT hydrogel. Optical microscopy images show that a ∼200 μm wide cleft was healed thoroughly upon NIR irradiation (Fig. 2d). Rather than a series of complicated and time-consuming steps such as disassembling, heating, and re-assembling,36 an in situ self-healing process can be achieved through the exposure to NIR or water, which effectively simplifies the mending process.
The dumbbell-shaped specimens (Fig. S2d†) were measured to evaluate the mechanical healing performance of the PVA/PDAP/MWCNT hydrogel, and the healing efficiency (η) can be quantitatively calculated using the recovery of fracture strain, according to the following equation:
 | (1) |
The introduction of PDAPs and borate bonds contributes to the formation of strong interfacial interactions between the filler and matrix and they can function as cross-linkers. Fig. 2e and f show the good mechanical properties of the as-prepared hydrogel in terms of high stress (83 kPa) and ultimate strain to fracture (440%). The η value of hydrogels in the presence of water was evaluated at ambient healing temperature (25 °C) for different periods of time (10 s, 30 s, 45 s and 300 s) after being cut completely. Both ultimate stress and strain to fracture recover back to about ∼93% of the pristine value in 300 s, which is much shorter than that required for the reported hydrogel samples (∼12 h).18,23 It was found that further elongation of the healing time does not lead to a higher η value, suggesting that an equilibrium state has been reached among borate bonds, hydroxyl groups of PVA crystallites and water.
Upon NIR irradiation, the PVA/PDAP/MWCNT hydrogel displayed a high photothermal conversion efficiency and raised the temperature of the matrix up to ∼120 °C in about 60 s (Fig. 1d), which allows the reversible melting/formation of the PVA hydrogel crystallites via switching on/off NIR light. With regard to this physical healing process, the healing efficiency was determined by the rearrangement behavior of the PVA molecular chains. A longer NIR exposure time results in higher healing temperature, leading to a larger melting region and higher probability to reform the PVA hydrogel crystallites, which contributes to better recovery of the targeted cracks. The ultimate stress can be fully recovered back to the pristine value after 60 s of NIR healing; the η value calculated based on the strain to fracture is 93%.
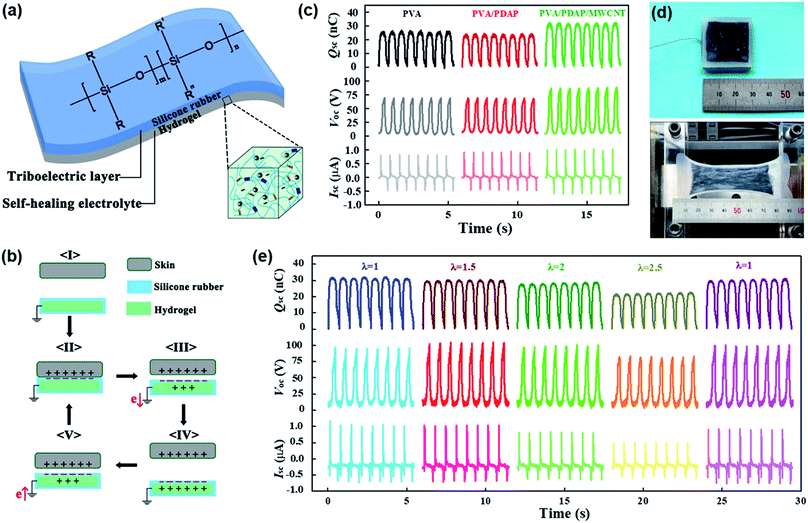
The general illustration and electrical output performance are displayed in Fig. 3. A typical HS-TENG mainly consists of two layers: PDAPs/MWCNTs are embedded in the PVA hydrogel substrate as a self-healing layer, and silicone rubber is cast on top of the hydrogel substrate as a triboelectric layer (Fig. 3a). Meanwhile, the MWCNTs mixed in the self-healing element can play the role of an electrolyte, establishing a good conductive network on the 2D plane for the HS-TENG. Here, the HS-TENG is designed to work in single-electrode mode which is more feasible in practical applications. The working mechanism of the HS-TENG is schematically illustrated in Fig. 3b. Driven by the physical contact of the skin, equivalent charges with opposite polarities are generated on the silicone rubber and human skin due to electrification (I). The silicone rubber is negatively charged meanwhile the skin is positively charged (II). Once the skin separates from the silicone rubber layer, positive charges are induced in the electrode layer (MWCNTs) and electrons would flow from the electrode to the ground through an external circuit, thus an electrical signal can be observed (III). When the skin is far enough from the silicone rubber layer, the induced positive charges on the electrode would fully balance the negative charges on the silicone rubber and the flow of electrons would stop (IV). Similarly, when the skin approaches the silicone rubber layer, electrons may flow from the ground to the electrode forming an opposite electrical signal (V). In such a circulation, an alternating current is generated. Several HS-TENGs (3 cm × 3 cm) with different compositions were applied for the electrical output measurements. The contact material is a Nylon film and the operating frequency is 1.5 Hz. The short-circuit transferred charge (Qsc), the open circuit voltage (Voc), and the short-circuit current (Isc) of both the PVA and PVA/PDAP HS-TENG are 26 nC, 70 V, and 0.8 μA, respectively (Fig. 3c). With the replacement of MWCNTs as an electrolyte layer, the output performance of the PVA/PDAP/MWCNT HS-TENG was increased by 26%, 35%, and 40%, respectively, compared to that of the above two compositions, indicating promising and practical applications.
Notably, the prepared PVA/PDAP/MWCNT HS-TENG exhibited very stable outputs over 500 cycles as shown in Fig. S3a.† Since the hydrogel was sealed with a layer (thickness = 1 mm) of silicone rubber during the preparation of the HS-TENG, water evaporation would not happen in the hydrogel of the HS-TENG. The output performances of a HS-TENG stored for 10 months were measured and it was found that they have similar values to those of a freshly prepared HS-TENG, as shown in Fig. S3b.† The electrical outputs of the HS-TENG under different stretched states were also measured (Fig. 3d). Compared with the initial state without strain (λ = 1), the Qsc, Voc, and Isc reached ∼32 nC, ∼95 V and ∼1 μA after being stretched for λ = 1.5 and 2, respectively. With the increase of strain (λ = 2.5), the values show a slight decline (∼20%) due to the resistance increment of the hydrogel electrode (Fig. S4b†). After recovering from the stretched states, the electrical output is comparable with the initial value, suggesting no degradation of the device. Although the resistance of the hydrogel is largely dependent on its stretch ratio (Fig. S4a†), the electrostatic induction, which is induced from a charged surface to an electrode, can be increased when the distance between the surface and electrode becomes shorter. Because a uniaxial strain reduces the thickness of the sample through the Poisson's effect, the induced output voltage was increased as reported by Lee et al.37
Once the PVA/PDAP/MWCNT HS-TENG device breaks down, the re-connection of the cracked parts can be accomplished by water (Fig. S5a†) and NIR (Fig. S6a†) induced self-healing processes. When the HS-TENG was broken, the output current value reduced by 50% because the HS-TENG was still working with only half of the original triboelectric area. The output current value started to increase when self-healing was triggered upon exposure to water spray (Fig. S5b†) or NIR light (Fig. S6b†). The electrical healing process can be accomplished in about 60 s using NIR light, which takes a similar time to that of the mechanical healing process. The stable electrical healing properties can be observed in Fig. S5c and S6c,† even after recovering 4 times upon both water spraying and NIR irradiation. Therefore, this HS-TENG taking advantage of MWCNTs as an electrolyte layer exhibits both outstanding electrical self-healing ability and electrical outputs. Table S1† presents the comparison of this HS-TENG with other self-healable TENGs. Except the PDMS-PU TENG, all listed TENGs show a complete recovery of Voc, but only the SH/CNT TENG (our previous research)12 and HS-TENG can realize a complete recovery of Qsc and Isc. In contrast to the other self-healable TENGs requiring a high temperature or long time (65 °C or 95 °C for hours), the HS-TENG shows much more efficient and environmentally friendly self-healing processes (under exposure to NIR light in 1 min and water spraying at 25 °C in 5 min). Since the HS-TENG can be healed by utilizing water spraying at ambient temperature (25 °C), it is possible to apply such a device as a real environmentally friendly wearable/portable power source.38 Additionally, NIR can be propagated to a long distance and has a good diffraction ability, implying a remotely controlled self-healing and its potential application under some harsh conditions such as upper air, abyssal sea, outer space and so on.
To obtain a maximum power transfer point of the HS-TENG, we varied the external load as shown in Fig. S7.† The output current decreased as the external resistance increased up to 2 GΩ and the maximum power density (750 mW m−2) was obtained with an external load of 500 MΩ. The capability of the HS-TENG in practical applications was explored, and several demonstrations are illustrated in Fig. 4. In order to power certain electronic devices, a circuit diagram was designed so that the alternating current could be converted to direct current through a rectifier (Fig. 4a). In such a setting, a capacitor with 22 μF was simulated with the frequency ranging from 0.5 to 2.5 Hz. Fig. 4b shows that the charging speed increases upon increasing the working frequency. It takes 800 s for the HS-TENG to charge the commercial capacitor to 1.7 V at a frequency of 2.5 Hz (Fig. 4c). The wearable self-powered system was demonstrated to be capable of harvesting sufficient mechanical energy from human motions to light 15 LEDs as displayed in Fig. 4d and e, and Movie S3.† And it took about 50 s for the HS-TENG to accomplish the charging and a digital watch could then be powered by the capacitor (Fig. 4f). The applicability of the HS-TENG as a soft energy device to harvest human motion energies was demonstrated (Fig. S8†). A HS-TENG device was attached on the elbow, and it can accommodate itself to the deformation of the elbow and remain attached conformally. By tapping the HS-TENG with various deformations, the rectified electricity (Fig. 4g and h) can charge commercial LEDs with sustainable energy, making it highly promising for powering many soft devices. The whole powering process is displayed in Movie S4.†
Conclusions
In summary, we have successfully produced an environmentally friendly, self-healable HS-TENG, which is achieved by introducing photothermally active polydopamine particles (PDAPs) and multiwalled carbon nanotube (MWCNTs), and water-active dynamic borate bonds into a PVA/agarose hydrogel. The wearable HS-TENG can be highly efficiently self-healed under both the exposure to near-infrared (NIR) light in 1 min and water spraying at 25 °C in 5 min according to physical and chemical healing mechanisms, respectively, which also indicates its promising in vivo applications as an implantable device due to the good tissue penetration ability of NIR light. The HS-TENG achieves high stretchability (∼400%), and mechanical (∼93%) and electrical (∼100%) healing efficiency, respectively. The applicability of the HS-TENG as a soft energy device to harvest human motion energies was demonstrated by tapping the HS-TENG with various deformations, and the rectified electricity can charge commercial LEDs with sustainable energy. With the required high flexibility/stretchability and healing ability, the HS-TENG is highly promising for powering many soft devices. This work provides a feasible technology to design TENGs with dual self-healing mode, which extends the reliability of TENGs for use in wearable electronics and potentially solves the energy issues of soft electronics.Experimental
Materials
Dopamine hydrochloric acid (DA·HCl, 99%) and agarose were purchased from Alfa Aesar Chemical Co., Ltd., China. Polyvinyl alcohol (PVA-124, degree of hydrolysis 99%, degree of polymerization 2400) was obtained from Sinopharm Chemical Reagent Co., Ltd. Sodium tetraborate decahydrate (Na2B4O7·10H2O, 99.5%) was bought from Energy Chemical. Multiwalled carbon nanotubes (MWCNTs) were purchased from Shenzhen Nanotech Port Co., Ltd. Silicone rubber (Ecoflex 00-30) was provided by Smooth-On, Inc. and used as-received.Preparation of polydopamine particles (PDAPs)31
NH3·H2O (3.75 mL, 28–30%), ethanol (60 mL, 99.5%) and H2O (135 mL) were added into a 250 mL three-necked flask and the mixture was stirred at a temperature of 30 °C for 30 min. DA·HCl (0.75 g) was added to 15 mL of H2O, and the obtained aqueous solution was transferred into the previous mixture, which was stirred for another 24 h at a temperature of 30 °C. Then, PDAPs were collected by centrifugation and washed with water three times.Fabrication of the self-healing hydrogel
PVA-124 (3.132 g), PDAPs (0.0783 g) and MWCNTs (0.14 g) were mixed with 12 mL of water. Then, the temperature was raised up to 90 °C and the mixture was stirred at this temperature for 30 min until a gel-like mixture was obtained. Then, Na2B4O7·10H2O solution (13 mL, 0.04 M) and agarose (0.28 g) were added directly to the gel-like mixture which was stirred for another 30 min at 95 °C. The prepared PVA/PDAP/MWCNT hydrogel was molded and subjected to freezing/thawing treatment, that is, initially cooling down to −20 °C for 1 h and then thawing at room temperature for 6 h. The freezing/thawing procedure was carried out 1–3 times to obtain the final product.Fabrication of the HS-TENG
Silicone rubber and a curing agent were mixed with a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) and the mixture was coated on a 31.0 mm × 31.0 mm mold up to a thickness of around 1.0 mm. Then, a piece of the PVA/PDAP/MWCNT hydrogel (30.0 mm × 30.0 mm × 1.0 mm) was placed on top of the previously coated silicone rubber. Then, another layer of silicone rubber with a thickness of around 1.0 mm was coated on the hydrogel to form a device with a sandwich structure.
1 (v/v) and the mixture was coated on a 31.0 mm × 31.0 mm mold up to a thickness of around 1.0 mm. Then, a piece of the PVA/PDAP/MWCNT hydrogel (30.0 mm × 30.0 mm × 1.0 mm) was placed on top of the previously coated silicone rubber. Then, another layer of silicone rubber with a thickness of around 1.0 mm was coated on the hydrogel to form a device with a sandwich structure.
Characterization
Tensile tests of the PVA/PDAP/MWCNT hydrogel were conducted on a Zwick 1445 tensile tester according to the Chinese standard GB/T2567-2008 using a cross-head speed of 2 mm min−1. All tensile data were obtained based on five samples with effective data points. A high-resolution JEOL scanning electron microscope (HR-SEM) was employed to study the morphology of hydrogels with different compositions. A Leica DMLM optical microscope was used to investigate the typical self-healing behavior of the PVA/PDAP/MWCNT hydrogel. The photothermal effect of PDAPs was recorded in real time using an Infrared Thermal Imager (Fotric 225s). A linear motor (Winnemotor, WMUC512075-06-X) was used to generate the contact and separation process controlling contact frequency. Nylon was attached to an acrylic plate that was mounted onto the linear motor. The HS-TENG was attached to another acrylic plate perpendicular to the direction of the motor. A programmable electrometer (Keithley 6514) was adopted to test the output performance, including Voc, Isc, and Qsc. For electrical connection of the device, copper foil was connected with the hydrogel-TENG to produce an electrical signal. In the quantitative test, the copper foil was connected to one terminal of a Keithley 6514, and the other terminal of Keithley 6514 was connected to the grounded terminal of the socket for grounding to form the testing circuit. The software platform was constructed on the basis of LabVIEW, which is capable of realizing real-time data acquisition control and analysis. The typical output performances were investigated by applying a linear motor at 1.5 Hz and 2 cm. The triboelectric area between the Nylon and HS-TENG was controlled to be the same as that of the stretched electrode. The input mechanical energy was acquired from the impact between the two pads. The linear motor was controlled by the software to move back and forward, which ensures the same distance and same frequency so that the input energy is the same.Author contributions
Q. G., G. L., Y. G. and W. T. fabricated materials and devices. D. B. and Y. G. characterized TENG performance. Q. G., Z. W., G. L., Y. L., J. W. X. S. and Y. P. analysed the data. Q. G., W. T., Z. W. and Y. P. designed materials. Q. G., Z. W., Y. P. and Z. Y. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (81870170, 51402203, 51703148, 61804103), Natural Science Foundation of Jiangsu Province of China (BK20170343), China Postdoctoral Science Foundation (2017M611901, 2017M610346), Science and Technology Program of Guangdong Province of China (2015B010131010), Guangdong Science and Technology Department (2017B030314026), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA535001, 14KJB150020), Fundamental Research Funds for the Central Universities (2232019D3-07), International Joint Laboratory for Advanced Fiber and Low-dimension Materials (18520750400), State Key Laboratory of Silicon Materials, Zhejiang University (SKL2018-03), Nantong Municipal Science and Technology Program (GY12017001), Joint International Research Laboratory of Carbon-Based Functional Materials and Devices and Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University (KJS1803), and Science and Technology Program of Suzhou (SYG201736). This work was also supported by the Collaborative Innovation Center of Suzhou Nano Science & Technology, Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), 111 Project, and Initial Research Funds for Young Teachers of Donghua University.References
- T. Liu, M. Liu, S. Dou, J. Sun, Z. Cong, C. Jiang, C. Du, X. Pu, W. Hu and Z. L. Wang, ACS Nano, 2018, 12, 2818–2826 CrossRef CAS PubMed.
- A. Chortos, J. Liu and Z. Bao, Nat. Mater., 2016, 15, 937–950 CrossRef CAS PubMed.
- T. B. H. Schroeder, A. Guha, A. Lamoureux, G. VanRenterghem, D. Sept, M. Shtein, J. Yang and M. Mayer, Nature, 2017, 552, 214–218 CrossRef CAS PubMed.
- S. Pan, H. Lin, J. Deng, P. Chen, X. Chen, Z. Yang and H. Peng, Adv. Energy Mater., 2015, 5, 1401438 CrossRef.
- M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588–610 RSC.
- J. Li, L. Kang, Y. Yu, Y. Long, J. J. Jeffery, W. Cai and X. Wang, Nano Energy, 2018, 51, 728–735 CrossRef CAS PubMed.
- Y. H. Jung, T. H. Chang, H. Zhang, C. Yao, Q. Zheng, V. W. Yang, H. Mi, M. Kim, S. J. Cho, D. W. Park, H. Jiang, J. Lee, Y. Qiu, W. Zhou, Z. Cai, S. Gong and Z. Ma, Nat. Commun., 2015, 6, 7170 CrossRef PubMed.
- Q. Zheng, Y. Zou, Y. Zhang, Z. Liu, B. Shi, X. Wang, Y. Jin, H. Ouyang, Z. Li and Z. L. Wang, Sci. Adv., 2016, 2, 1501478 CrossRef PubMed.
- Z. L. Wang, Nano Energy, 2018, 54, 477–483 CrossRef CAS.
- C. Wu, A. C. Wang, W. Ding, H. Guo and Z. L. Wang, Adv. Energy Mater., 2019, 9, 1802906 CrossRef.
- W. Hu, X. Wei, L. Zhu, D. Yin, A. Wei, X. Bi, T. Liu, G. Zhou, Y. Qiang, X. Sun, Z. Wen and Y. Pan, Nano Energy, 2019, 57, 600–607 CrossRef CAS.
- Q. Guan, Y. Dai, Y. Yang, X. Bi, Z. Wen and Y. Pan, Nano Energy, 2018, 51, 333–339 CrossRef CAS.
- Z. L. Wang, J. Chen and L. Lin, Energy Environ. Sci., 2015, 8, 2250–2282 RSC.
- Z. Wen, J. Fu, L. Han, Y. Liu, M. Peng, L. Zheng, Y. Zhu, X. Sun and Y. Zi, J. Mater. Chem. C, 2018, 6, 11893–11902 RSC.
- S. Chen, T. Huang, H. Zuo, S. H. Qian, Y. F. Guo, L. J. Sun, D. Lei, Q. L. Wu, B. Zhu, C. L. He, X. M. Mo, E. Jeffries, H. Yu and Z. W. You, Adv. Funct. Mater., 2018, 28, 1805108 CrossRef.
- T. P. Huynh, P. Sonar and H. Haick, Adv. Mater., 2017, 29, 1604973 CrossRef PubMed.
- J. Deng, X. Kuang, R. Liu, W. Ding, A. C. Wang, Y. C. Lai, K. Dong, Z. Wen, Y. Wang, L. Wang, H. J. Qi, T. Zhang and Z. L. Wang, Adv. Mater., 2018, 30, 1705918 CrossRef PubMed.
- X. Pu, M. Liu, X. Chen, J. Sun, C. Du, Y. Zhang, J. Zhai, W. Hu and Z. L. Wang, Sci. Adv., 2017, 3, 1700015 CrossRef PubMed.
- X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed.
- S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding and X. Chen, Adv. Sci., 2018, 5, 1700527 CrossRef PubMed.
- T. An and W. Cheng, J. Mater. Chem. A, 2018, 6, 15478–15494 RSC.
- X. Zhao, F. Chen, Y. Li, H. Lu, N. Zhang and M. Ma, Nat. Commun., 2018, 9, 3579 CrossRef.
- J. Sun, X. Pu, M. Liu, A. Yu, C. Du, J. Zhai, W. Hu and Z. L. Wang, ACS Nano, 2018, 12, 6147–6155 CrossRef CAS PubMed.
- J. Kang, D. Son, G. N. Wang, Y. Liu, J. Lopez, Y. Kim, J. Y. Oh, T. Katsumata, J. Mun, Y. Lee, L. Jin, J. B. Tok and Z. Bao, Adv. Mater., 2018, 30, 1706846 CrossRef PubMed.
- K. Parida, V. Kumar, W. Jiangxin, V. Bhavanasi, R. Bendi and P. S. Lee, Adv. Mater., 2017, 29, 1702181 CrossRef PubMed.
- S. C. G. Leeuwenburgh, N. De Belie and S. van der Zwaag, Adv. Mater. Interfaces, 2018, 5, 1800736 CrossRef.
- Y. Tsou, X. Zhang, X. Bai, H. Zhu, Z. Li, Y. Liu, J. Shi and X. Xu, Adv. Funct. Mater., 2018, 28, 1802607 CrossRef.
- Y. Yang, J. He, Q. Li, L. Gao, J. Hu, R. Zeng, J. Qin, S. X. Wang and Q. Wang, Nat. Nanotechnol., 2018, 14, 151–155 CrossRef PubMed.
- Z. Wang, G. An, Y. Zhu, X. Liu, Y. Chen, H. Wu, Y. Wang, X. Shi and C. Mao, Mater. Horiz., 2019 10.1039/c8mh01208c.
- L. Yang, Z. Wang, G. Fei and H. Xia, Macromol. Rapid Commun., 2017, 38, 1700421 CrossRef PubMed.
- Y. Li, Y. Xie, Z. Wang, N. Zang, F. Carniato, Y. Huang, C. M. Andolina, L. R. Parent, T. B. Ditri, E. D. Walter, M. Botta, J. D. Rinehart and N. C. Gianneschi, ACS Nano, 2016, 10, 10186–10194 CrossRef CAS PubMed.
- P. W. Barone, S. Baik, D. A. Heller and M. S. Strano, Nat. Mater., 2005, 4, 86–92 CrossRef CAS PubMed.
- N. W. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600–11605 CrossRef CAS PubMed.
- H. K. Moon, S. H. Lee and H. C. Choi, ACS Nano, 2009, 3, 3707–3713 CrossRef CAS PubMed.
- S. Shen, Y. Chao, Z. Dong, G. Wang, X. Yi, G. Song, K. Yang, Z. Liu and L. Cheng, Adv. Funct. Mater., 2017, 27, 1700250 CrossRef.
- Q. T. Li, M. J. Jiang, G. Wu, L. Chen, S. C. Chen, Y. X. Cao and Y. Z. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 20797–20807 CrossRef CAS PubMed.
- Y. Lee, S. H. Cha, Y.-W. Kim, D. Choi and J.-Y. Sun, Nat. Commun., 2018, 9, 1804–1811 CrossRef PubMed.
- Y. Yang, N. Sun, Z. Wen, P. Cheng, H. Zheng, H. Shao, Y. Xia, C. Chen, H. Lan, X. Xie, C. Zhou, J. Zhong, X. Sun and S. T. Lee, ACS Nano, 2018, 12, 2027–2034 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ta02711d |
| This journal is © The Royal Society of Chemistry 2019 |