Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems
Abstract
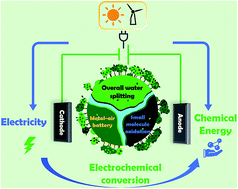
There remains a grand challenge to develop sustainable and pollution-free energy to replace the current dominant but gradually “depleting” fossil fuels. Electrochemical energy conversion presents a promising “bridge” to mitigate energy shortage issues and minimize the ecological implications by synergy with the ever-increasing sustainable energies (e.g., wind and solar). It thus calls for exploring high-activity, low-cost, and long-durability electrocatalysts to facilitate the electrochemical reactions involved in electrochemical energy conversion systems. This review focuses on summarizing the recent progress in the development of bifunctional electrocatalysts for several important electrochemical redox reactions which are critical to implement conversion between electrical energy and chemical energy, especially for bifunctional electrocatalysts applied in a single electrochemical conversion system, including water electrolyzers with the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), zinc–air batteries with the OER and oxygen reduction reaction (ORR), and fuel cells with small molecule (e.g., glucose, urea, and hydrazine hydrate) oxidation reactions (MOR) and the ORR. Special emphasis has been placed on investigating the progress of electrocatalyst synthesis and strategies for improving the electrocatalytic performance and the associated devices' performance in terms of activity, stability, power density etc. We also put forward the major challenges and prospects in the development of bifunctional electrocatalysts for potential applications in a variety of energy devices.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...