Controllable CO2 conversion in high performance proton conducting solid oxide electrolysis cells and the possible mechanisms†
Abstract
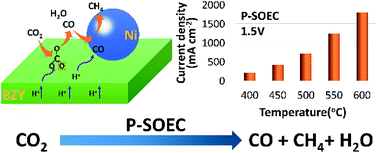
To solve the increasing greenhouse problem and to achieve sustainable carbon cycling, effective conversion of CO2 through chemical or electrochemical ways is key. In this study, efficient and controllable conversion of CO2 mainly to CO and CH4 has been demonstrated in a proton conducting solid oxide electrolysis cell (P-SOEC) using BaZr0.8Y0.2O3−δ (BZY) as the electrolyte and SrEu2Fe1.8Co0.2O7−δ as the anode, in which an excellent current density of 1.23 A cm−2 at 1.5 V was achieved at 550 °C and 100 hours of smooth operation is demonstrated. Compared with the pure steam electrolysis, impedance spectral investigations indicate that the presence of CO2 in the cathode actually accelerates the electrode reactions, in contrast with that in a regular O-SOEC. This may be attributed to the higher adsorption of CO2 and more effective conversion of protons over the BZY electrolyte. With the increase of electrolysis current, formation of both CO and CH4 are enhanced, contradictory to the deduction based on thermodynamic calculations in which the concentration of CH4 increases while that of CO reduces. In situ Raman and in situ diffuse reflectance FTIR spectroscopy (in situ DRIFTS) was conducted, and reaction routes for CO2 were then proposed. Continuously replenished protons, which steadily and efficiently react with CO32− to form–OCO– and finally CO, are suggested to play a critical role in the conversion of CO2 and the formation of CO in the P-SOEC. Our results shed new light on future effective conversion of CO2.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...