P2-type Na2/3Ni1/3Mn2/3O2 as a cathode material with high-rate and long-life for sodium ion storage†
Abstract
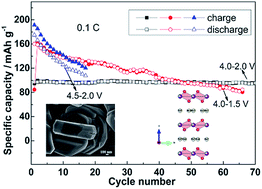
Layered P2-type Na2/3Ni1/3Mn2/3O2 was successfully synthesized through a facile sol–gel method and subsequent heat treatment. Resulting from different phase transformation and sodium ion diffusion rates, its electrochemical performance is highly related to the cut-off voltage and the electrolyte used. When the cut-off voltage is set up to 4.5 V or lowered to 1.5 V, capacity fade happens due to the occurrence of P2–O2 transformation and electrolyte decomposition or the redox reaction of the Mn4+/Mn3+ ionic pair and P2–P2′ transformation. The electrode maintained 89.0 mA h g−1 with good cycling stability and excellent structural preservation between 4.0 and 2.0 V. The capacity retention is 71.2% even after 1200 cycles at 10C. It can be expected that P2-type Na2/3Ni1/3Mn2/3O2 is very promising as a cathode material for sodium ion batteries.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...