Intercalated complexes of 1T′-MoS2 nanosheets with alkylated phenylenediamines as excellent catalysts for electrochemical hydrogen evolution†
Abstract
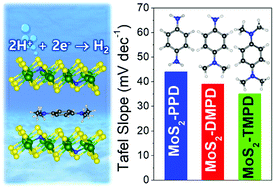
Two-dimensional layered MoS2 has recently been considered as an excellent catalyst for the water-splitting hydrogen evolution reaction (HER). Herein, we synthesize 1T′ phase MoS2 that was intercalated with a series of alkylated p-phenylenediamines (PDs). The substituted N atoms produced S vacancies, leading to a composition of MoS2−2xNx (x = 0.1). The more abundant methyl groups induce a larger charge transfer, resulting in excellent HER performance: for tetramethyl PD, the overpotential is 0.15 V at 10 mA cm−2 with a Tafel slope of 35 mV dec−1. The catalytic activity of the complexes depends on the concentration of the intercalated molecules, showing an optimum at a concentration of 8 mol%. First-principles calculations showed that the intercalated complexes (1T′ phase) having N atom–S vacancy (N–VS) pairs are stabilized by a large charge transfer from the PD molecules that is enhanced by the methyl groups (i.e., 0.40e–0.84e per molecule at 6.25 mol% intercalation). The charge transfer increases the density of states at and just above the Fermi level, thereby increasing the electron concentration at low cathodic bias. The active sites for the Volmer reaction are found to be N atoms in the proximal N–VS pairs. The activation barrier for the Heyrovsky reaction becomes higher at higher concentrations of the intercalants, suggesting that the experimental HER performance is also kinetically controlled.


 Please wait while we load your content...
Please wait while we load your content...