Uniform Pd0.33Ir0.67 nanoparticles supported on nitrogen-doped carbon with remarkable activity toward the alkaline hydrogen oxidation reaction†
Abstract
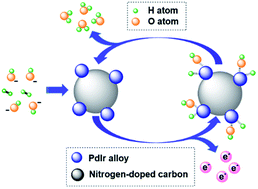
Highly efficient non-Pt electrocatalysts for the alkaline hydrogen oxidation reaction (HOR) are required to enable complete replacement of Pt in hydroxide exchange membrane fuel cells (HEMFCs). Herein, we report a facile synthesis of a series of 2.4–2.9 nm Pd1−xIrx (x = 0.33, 0.50, 0.67, 0.75, 0.80, 0.91) alloy nanoparticles (NPs) evenly distributed on nitrogen-doped carbon (N-C) via simple chemical reduction of aqueous metallic complexes by sodium borohydride (NaBH4) in the absence of surfactants. The Ir component of alloy NPs and the nitrogen dopants of the carbon matrix contribute to the particle size control and uniform distribution. Remarkably, the resultant Pd0.33Ir0.67/N-C exhibits an exceptional alkaline HOR activity, measured as mass specific exchange current density (j0,m), that is 1.4 times that of commercial Pt/C. CO stripping shows that Pd0.33Ir0.67/N-C has an electrochemical active surface area (ECSA) of 106 m2 gmetal−1 that is 1.2 times that of commercial Pt/C, partially explaining the increased activity. Furthermore, density functional theory (DFT) demonstrates an appropriate strength of hydrogen binding of Pd0.33Ir0.67, which is consistent with cyclic voltammetry (CV) measurements. In addition, DFT shows that Pd0.33Ir0.67 possesses the highest oxophilic property among all of the Pd1−xIrx electrocatalysts. We conclude that the high ECSA, appropriate strength of hydrogen binding, and the strong oxophilic property collectively account for the remarkable activity of Pd0.33Ir0.67/N-C. The latter two factors should be closely correlated with the electronic effect between Pd and Ir as evidenced by X-ray photoelectron spectroscopy (XPS). A single cell fabricated with Pd0.33Ir0.67/N-C as the anode approaches a peak power density of 514 mW cm−2 that is 1.3 times that of commercial Pt/C. This study demonstrates the substitution of commercial Pt/C with a non-Pt electrocatalyst at the anode of the single cell of HEMFCs with enhanced performance.


 Please wait while we load your content...
Please wait while we load your content...