Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(vi)†
Abstract
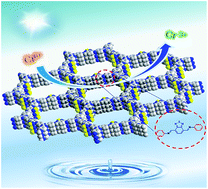
Covalent organic frameworks (COFs) have received increasing research interest as an emerging class of crystalline and porous polymers. Herein, we prepared two new benzothiadiazole (BT) functionalized COFs (i.e. TPB-BT-COF and TAPT-BT-COF), which exhibit good crystallinity, high porosity, and excellent stability in harsh conditions. Their applications for photoreduction of Cr(VI) species under visible light irradiation were investigated. Over 99% Cr(VI) was reduced by utilizing TPB-BT-COF as catalyst without any sacrificial agents or additional pH adjustment. The photocatalytic rate of TPB-BT-COF is faster than that of TAPT-BT-COF, which can be attributed to more negative conduction band and narrower bandgap. Our results indicate that co-condensation of electron deficient units into the COF skeleton is conducive to efficient separation of photoexcited electrons and holes, which in turn leads to superior photocatalytic activities.


 Please wait while we load your content...
Please wait while we load your content...