Chemical stimulus-responsive supramolecular hydrogel formation and shrinkage of a hydrazone-containing short peptide derivative†
Abstract
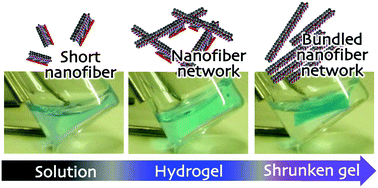
Artificial supramolecular nanostructures showing transient properties have attracted significant attention in recent years. New discoveries in this area may provide insights into a better understanding of the sophisticated organization of complex biomolecular systems. Nevertheless, research concerning such materials is still limited. Better knowledge of the chemical reactivity and corresponding molecular transformations of self-assembling molecules, which guide their assembly/disassembly, may provide an opportunity to construct transient supramolecular nanostructures capable of showing chemical stimulus responsiveness. Herein, we report a short peptide derivative containing a hydrazone bond, which shows transient hydrogel formation (no only sol-to-gel but also gel-to-shrunken gel phase transition) accompanied by continuous transformation and growth of supramolecular nanostructures triggered by hydrazone–oxime exchange reaction in response to hydroxylamine. Such controlled shrinkage behavior of supramolecular hydrogels in response to specific chemical stimuli has rarely been explored compared with conventional polymer hydrogel systems.
- This article is part of the themed collection: Peptide Soft Materials


 Please wait while we load your content...
Please wait while we load your content...