 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure and dynamics of hagfish mucin in different saline environments†
Katerina
Rementzi
ab,
Lukas J.
Böni
 c,
Jozef
Adamcik
c,
Jozef
Adamcik
 c,
Peter
Fischer
c,
Peter
Fischer
 *c and
Dimitris
Vlassopoulos
*c and
Dimitris
Vlassopoulos
 *ab
*ab
aFORTH, Institute of Electronic Structure & Laser, N. Plastira 100, 70013 Heraklion, Greece. E-mail: dvlasso@iesl.forth.gr
bDepartment of Materials Science & Technology, University of Crete, P.O. Box 2208, 71003 Heraklion, Greece
cInstitute of Food, Nutrition and Health, ETH Zürich, 8092 Zürich, Switzerland. E-mail: peter.fischer@hest.ethz.ch
First published on 14th October 2019
Abstract
The defense mechanism of hagfish against predators is based on its ability to form slime within a few milliseconds. Hagfish slime consists of two main components, namely mucin-like glycoproteins and long protein threads, which together entrap vast amounts of water and thus form a highly dilute hydrogel. Here, we investigate the mucin part of this hydrogel, in particular the role of the saline marine environment on the viscoelasticity and structure. By means of dynamic light scattering (DLS), shear and extensional rheology we probe the diffusion dynamics, the flow behavior, and the longest filament breaking time of hagfish mucin solutions. Using DLS we find a concentration-independent diffusion coefficient – characteristic for polyelectrolytes – up to the entanglement regime of 0.2 mg ml−1, which is about ten times higher than the natural concentration of hagfish mucin in hagfish slime. We also observe a slow relaxation process associated with clustering, probably due to electrostatic interactions. Shear rheology further revealed that hagfish mucin possesses pronounced viscoelastic properties at high concentrations (3 mg ml−1), showing that mucin alone achieves mechanical properties similar to those of natural hagfish slime (mucins and protein threads). The main effects of added seawater salts, and predominantly CaCl2 is to reduce the intensity of the slow relaxation process, which suggests that calcium ions lead to an ionotropic gelation of hagfish mucins.
I. Introduction
Marine dwelling hagfishes produce vast amounts of slime when attacked.1 The slime serves as an immediate defense mechanism against potential predators2 and acts by clogging their mouth and gills.3 A glandular white secretion, so-called exudate, is released from the ventrolateral pores of hagfish into the surrounding seawater. The exudate consists of two main components, namely mucin vesicles and protein threads packed into skeins (Fig. 1a). A skein looks like a microscopic ‘ball-of-wool’ and is made of a single coiled-up protein thread that is up to 30 cm long and 1–3 μm in diameter. When skeins are ejected from the slime glands into the seawater, they unravel and deploy their long intermediate filament bundle fiber, creating a cohesive fiber network of protein threads.4,5 The other main component, the mucin vesicles contain mucin-like glycoproteins, which are negatively charged by sulphonated side groups.6–8 Once in contact with seawater, the mucin vesicles swell, rupture, and discharge their mucin.9 The hydrated mucin and the network of unraveled skeins synergistically form hagfish slime (Fig. 1b). Hagfish slime is in many ways an exceptional natural hydrogel. It can physically confine up to 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times its own weight in water given its sieve-like structure made by the protein threads and mucin. Also, it forms within milliseconds in high osmolarity seawater, is coherent, elastic yet extremely soft,10,11 which is a unique combination of features for hydrogels found in nature.
000 times its own weight in water given its sieve-like structure made by the protein threads and mucin. Also, it forms within milliseconds in high osmolarity seawater, is coherent, elastic yet extremely soft,10,11 which is a unique combination of features for hydrogels found in nature.
The fact that a charged gel with a complex network structure rapidly deploys in a strongly charge-screening environment as in seawater (ionic strength of about 0.7 M12) is intriguing and has led to studies that investigated the effects of ionic strength and ionic composition. The main areas of focus comprised the effects of salts on ex vivo stabilization of hagfish exudate,8,13–15 skein unraveling,16,17 mucin vesicle swelling and rupture,9,16–18 swelling and hydration of skeins19 and reconstituted biomimetic materials made from slime thread proteins,20 and whole hagfish slime functionality.17 These studies show (i) that a high osmolarity of about 800 mOsmol l−1 and higher achieved by divalent anions (sulfate, citrate, phosphate) stabilizes hagfish exudate,8,14,15 (ii) that seawater assists the dissolution of a glue, which holds the native skeins together and mediates unraveling,16 (iii) that protein threads and materials made from those protein swell less in high osmolarity environments,19,20 (iv) that viscosities of mucin solutions are lower in seawater than in deionized water,11,17 and (v) that swelling and rupture of hagfish mucin vesicles requires the presence calcium ions when the ionic strength of the solution is >100 mM.18 Especially calcium ions seem to exert critical roles in hagfish slime functionality.17 First, calcium ions are considered to keep the polyanionic mucin molecules within the vesicles in a condensed state before swelling.9,21 Second, Ca2+–ions were shown to be important for mucin vesicle decondensation by activating Ca2+-mediated transporter proteins in the vesicle membrane, which facilitate water influx and swelling of mucin vesicles.18 And third, Ca2+–ions were found to be crucial in realizing a functional slime network. Calcium seems to be required for an ionotropic gelation of hagfish mucin, forming a non-covalently stabilized mucin network inside the slime that retains the water.17 Ionotropic gelation, also called polyelectrolyte complexation is based on the ability of polyelectrolyte molecules to form hydrogels in the presence of counterions even below the entanglement concentration.22–25
Although previous works studied hagfish mucin vesicles and hagfish mucin in stabilization solutions and different saline environments, still little is known about the fundamental interactions of hagfish mucin with salts and especially calcium ions. In this work we investigated the structure and dynamics of hagfish mucin over broad length and time domains and focused especially on the effect of different salt environments. Using atomic force microscopy, we first elucidate the size and morphology of single mucin vesicles and mucin molecules. Advanced light scattering and extensional rheology were then used to explore the diffusion and disentanglement kinematics of hagfish mucin over a broad range of concentrations and thus determine an entanglement concentration, which was found to be much higher than the natural concentration of hagfish mucin. In a last part, we investigate the effect of calcium ions on the intra- and intermolecular diffusion of mucin molecules and show how they affect the rheological properties.
II. Materials & methods
Sample collection
Sample collection was conducted according to a protocol described by Böni et al.13 In brief, Atlantic hagfish (Myxine glutinosa) were kindly fished in the Fjords of Ålesund by the staff of the Atlanterhavsparken (Norway). A mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 of clove bud oil (Sigma-Aldrich) and ethanol was used for hagfish anesthetization. Sedated hagfish were placed on a dissection board and plotted dry. Subsequently, electrical stimulation (HPG1, Velleman Instruments, 80 Hz, 8–18 V) was used on the ventrolateral side of the sedated hagfish to contract the local muscles and thus expel the exudate from the slime pores. A polished spatula was used to remove the secreted exudate, which was immediately stabilized in a high osmolarity citrate-PIPES (CP) buffer consisting of 0.9 M sodium citrate and 0.1 M PIPES at pH 6.7, 0.02% NaN3 and a protease inhibitor. Samples were stored at 4 °C. After sampling hagfish were allowed to recover and subsequently release back to the sea. Sampling procedure was performed in accordance with the approved ethical application by the Forsøksdyrutvalget (FOTS ID 6912).
9 of clove bud oil (Sigma-Aldrich) and ethanol was used for hagfish anesthetization. Sedated hagfish were placed on a dissection board and plotted dry. Subsequently, electrical stimulation (HPG1, Velleman Instruments, 80 Hz, 8–18 V) was used on the ventrolateral side of the sedated hagfish to contract the local muscles and thus expel the exudate from the slime pores. A polished spatula was used to remove the secreted exudate, which was immediately stabilized in a high osmolarity citrate-PIPES (CP) buffer consisting of 0.9 M sodium citrate and 0.1 M PIPES at pH 6.7, 0.02% NaN3 and a protease inhibitor. Samples were stored at 4 °C. After sampling hagfish were allowed to recover and subsequently release back to the sea. Sampling procedure was performed in accordance with the approved ethical application by the Forsøksdyrutvalget (FOTS ID 6912).
Sample preparation
The vesicle stock suspension was prepared according to the protocol of Salo et al.8 In brief, exudate stabilized in high osmolarity buffer was filtered through a series (60 and 20 μm) of nylon mesh filters (Merck) to separate the small mucin vesicles from the skeins. To obtain a high mucin vesicle concentration, the filtrate was centrifuged at 2000 g for 10 minutes and the supernatant discarded. The mucin content of the vesicle suspension was determined in triplicates by dialysis (25 kDa MWCO, SpectraPor, USA), i.e. dialyzing 0.5 ml of the vesicle suspension against three batches of Milli-Q water (12 h each) and subsequent freeze drying to determine the dry weight. Hagfish mucin solutions were prepared by mixing stabilized mucin vesicles with Milli-Q water or solutions containing the salts of interest in a 2 ml Eppendorf tube, sloshing it heads over eight times. Mucin solutions containing maximum 1% of the vesicle stock suspension were prepared. If lower percentages of vesicle stock suspensions were used, the final mucin solution was adjusted for ionic strength by adding the required amount of CP buffer to achieve 1% CP buffer in the solution. Artificial seawater (ASW) was prepared according to a recipe of Kester et al.12 All salts were obtained from Sigma. For the dissolved salts a complete dissociation was assumed.Microscopy
Atomic force microscopy (AFM) for vesicle imaging was performed on a Cypher (Asylum Research, USA) and on a Bruker FastScan microscope (Bruker, USA) for mucin imaging. Images were acquired in tapping mode in air and water, using BioLevers Mini (Olympus, Japan) to image the vesicles and RTESPA 150 cantilevers (Bruker, USA) for the mucin. To prepare the vesicle sample, a droplet of vesicles in CP buffer was put on a silicon wafer and let to rest for about three hours so the vesicles could sink and weakly stick to the surface. For the mucin, repeatedly washed vesicles stock suspension was dialyzed (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kDa MWCO, SpectraPor, USA) Milli-Q (three batches, 12
kDa MWCO, SpectraPor, USA) Milli-Q (three batches, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h each). The dialyzed samples were filled into 15 ml Falcon tubes and diluted with Milli-Q to get a mucin concentration of roughly 0.2 mg ml−1. Droplet of mucin solution (mucin dialysed against Milli-Q or see water) was deposited on freshly cleaved mica, adsorbed for 2 min, washed with Milli-Q water and dried by air. Cryo-Scanning electron microscopy (cryo-SEM) was performed on a LEO 1530 (Carl-Zeiss SMT AG). Vesicles in CP buffer were snap frozen with a high-pressure freezer (Bal-Tec HPM100), freeze-fractured (Bal-Tec BAF060), sputter coated (SCD 050 sputter coater, Bal-Tec) with a 3 nm thick platinum layer and then imaged.
h each). The dialyzed samples were filled into 15 ml Falcon tubes and diluted with Milli-Q to get a mucin concentration of roughly 0.2 mg ml−1. Droplet of mucin solution (mucin dialysed against Milli-Q or see water) was deposited on freshly cleaved mica, adsorbed for 2 min, washed with Milli-Q water and dried by air. Cryo-Scanning electron microscopy (cryo-SEM) was performed on a LEO 1530 (Carl-Zeiss SMT AG). Vesicles in CP buffer were snap frozen with a high-pressure freezer (Bal-Tec HPM100), freeze-fractured (Bal-Tec BAF060), sputter coated (SCD 050 sputter coater, Bal-Tec) with a 3 nm thick platinum layer and then imaged.
Dynamic light scattering
Dynamic light scattering (DLS) measurements were carried out using a ALV-5000 system (ALV, Germany) operating with a polarized monochromatic laser beam at a wavelength of λ = 532 nm. The temperature was controlled using a recirculating fluid bath (15 °C < T < 50 °C). The scattered intensity was detected by the photomultiplier and analyzed in a correlator yielding eventually information about the relaxation dynamics of the measured object. The scattering angles between incident and scattered light (25° < θ < 150°) define the scattering wavevector q = (4πn/λ) sin(θ/2), which is the inverse length scale probed (0.00681 nm−1< q < 0.03039 nm−1), where n is the refractive index. The intensity correlation function is expressed as: | (1) |
| g(2)(τ) = B + β|g(1)|2 | (2) |
 | (3) |
 | (4) |
 | (5) |
Shear and extensional rheology
Oscillatory shear and creep measurements were conducted with a MCR501 rheometer from Anton-Paar (Austria) using a cone-plate geometry with a diameter of 50 mm and a cone angle of 1° (truncation of 102 μm). Measurements were performed at 20 °C. For extensional rheometry the CaBER 1 rheometer (Thermo Haake, Thermo Fisher Scientific) was used. Cylindrical plates with diameter of 6 mm were used. An initial gap of 3 mm and a final gap of 12 mm were chosen, using a strike time of ts = 50 ms. The sample was loaded with a micropipette (80 μL) on the bottom plate, an initial step strain was applied and as a result a liquid filament was formed. Within this capillary an extensional flow field establishes. For Newtonian liquids the capillary diameter decreases linearly with time. For viscoelastic fluids such as polymer solutions large non-Newtonian elastic stresses can grow during the transient elongational stretching process. If these elastic stresses start to dominate during capillary thinning and the corresponding viscous stresses become negligible, an elasto-capillary force balance predicts that the radius of the filament decays exponentially in time:30 | (6) |
III. Results
Vesicle and mucin structure
Fig. 2a shows an atomic force microscopy (AFM) image of a mucin vesicle taken in citrate-PIPES (CP) buffer. The inlet depicts the height profile of the vesicle along the longest axis. An isolated vesicle has an oval-like shape and resembles a disk (3.5 μm long and 200–400 nm high) and shows tiny bumps on its surface, of which the origin and function are yet unknown. The oval disk shape and the bumps are also confirmed in cryo-SEM images (Fig. 2b). Additional cryo-Sem images can be found in Fig. S1 (ESI†). | ||
| Fig. 2 (a) AFM image taken in CP buffer solution and (b) cryo-SEM image of a hagfish mucin vesicle stabilized in CP buffer solution. | ||
When in contact with water, mucin vesicles swell and release the mucin macromolecules. Fig. 3a displays AFM images of native mucin forming long bundled fibrillar structures. Fig. 3b and c show AFM image of the individual chains of mucin dialyzed in Milli-Q and sea water revealing the different morphology under different ionic conditions.
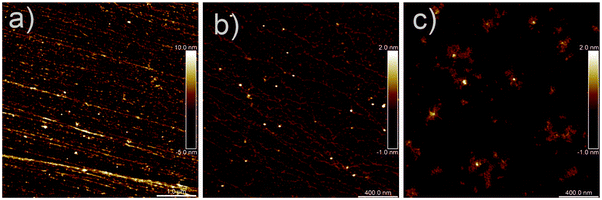 | ||
| Fig. 3 AFM image of (a) native mucin, (b) mucin dialyzed in Milli-Q water and (c) mucin dialyzed in seawater. | ||
Dynamics and rheology of mucin at different concentrations
Dynamic light scattering (DLS) measurements probe the internal dynamics of hagfish mucin. As shown by AFM images above, hagfish mucins are very large filamentous macromolecules with lengths L of the order of micrometers, resulting in qR > 1. The analysis of the intermediate scattering (time autocorrelation) functions of mucin solutions at various concentrations resulted in a very large observed size at q = 0.01204 nm−1 (Fig. 4a), which is assigned to the hydrodynamic correlation length ξH (mesh size of the network). Under the present conditions the investigated samples are ergodic as confirmed by the large (and only weakly changing) total amplitude of the intermediate scattering functions and the fact that slow modes relax within the experimental time window. The highest concentration of 3.47 g ml−1 may be at the onset of non-ergodicity but the fast relaxation dynamics, which is of concern in this work, is virtually unaffected. This is typical situation in weak gels, which have been studied with conventional, time-averaged DLS.26,27,31 The non-ergodic regime is beyond the scope requires spartical averaging of the intermediate scattering function.27,32 In Fig. 4b the apparent diffusion coefficient for a mucin concentration of cmucin = 0.08 mg ml−1 is shown. From the measurement the apparent diffusion coefficient Dapp(q → 0) = 4.7 × 10−9 cm−2 s−1 and a respective hydrodynamic correlation length ξH = 455 nm were extracted. Apparent diffusion coefficients for a wider range of mucin concentrations (4.3 × 10−4–3.47 mg ml−1) showed a similar trend as in Fig. 4b and are summarized in Fig. S2 (ESI†). Furthermore, the scattering intensity for all mucin concentrations depends strongly and virtually identically on the scattering wavevector q (Fig. S3, ESI†), pointing to large length scales with the internal structure of mucin being independent of concentration.33 | ||
| Fig. 4 DLS on the dynamics of hagfish mucin in Milli-Q water. (a) Intermediate scattering functions (C(q, t) = g(2) − 1) at different mucin concentrations in water at θ = 45° (q = 0.01204 nm−1). (b) Extracted apparent diffusion coefficient Dapp(q → 0) for cmucin = 0.08 mg ml−1 (line to guide the eye, representative errors are summarized in Fig. 5). | ||
In Fig. 5 the apparent diffusion coefficient as a function of mucin concentration is summarized. Starting from a concentration of 0.2 mg ml−1 the diffusion coefficients start to decline. This indicates that the mucin entanglement concentration in Milli-Q is around 0.2 mg ml−1. Fig. 5 also shows that the addition of various salts added to the mucin solution does not substantially affect the diffusion coefficient at the reference concentration of 0.08 mg ml−1. The low q region at q → 0 is not detectable and in order to estimate the I(q) we used the Debye plot (Fig. S4, ESI†) and the reduced total intensity (Fig. S5, ESI†). The intensity remains constant until it reaches the concentration of 0.2 mg ml−1, which is in a good agreement with the diffusion coefficient against the same range of concentrations.
At high mucin concentrations (cmucin = 3 mg ml−1), oscillatory shear data (Fig. 6a) suggest a viscoelastic mucin network, which shows a predominant elastic modulus G′ and weakly frequency dependent moduli, i.e. indicating a typical soft gel-like behavior. To access the low-frequency crossover of the viscoelastic moduli, a step stress creep test at shear stress of σ = 0.04 Pa was applied for 21 h and reveals a stress relaxation process of hagfish mucin at the crossover frequency of ω = 10−5 rad s−1 or a stress relaxation time of about 20 hours. In Fig. 6b capillary breakup extensional rheometry (CaBER) measurements are shown. The normalized diameter of the formed capillaries decays as function of time for four mucin concentrations. The curves show a pronounced elasto-capillary breakup regime, supporting the viscoelastic behavior observed in oscillatory shear flow, also at lower concentrations. From the elasto-capillary breakup, an extensional relaxation time λ and the apparent modulus Gapp can be extracted, which are plotted together with the filament break-up time tB in Fig. 6c. The apparent modulus Gapp in extension is consistent with the storage modulus G′ measured in shear.
Effects of sea water salts on hagfish mucin
The effect of salts and salt concentration on hagfish mucin was studied using artificial seawater (ASW). Typical intermediate scattering (time autocorrelation) functions for mucin solutions in ASW are depicted in Fig. 7a where a dominant fast relaxation process (10−3 s < t < 10−1 s) is observed, along with a slow relaxation process (10−1 s < t < 101 s). The investigated samples are ergodic as in the context of Fig. 4a. The analysis of the fast relaxation process reveals an apparent diffusion coefficient of Dapp(q → 0) = 5.32 × 10−9 cm2 s−1 (Fig. 7b). Fig. 7c shows the filament thinning CaBER experiment of mucin in dilutions of ASW. With increasing ASW concentrations, the capillary lifetime reduces. This suggests that in the absence of seawater salts hagfish mucin possesses a higher viscoelasticity, which was similarly shown for the viscosity of hagfish mucin.11,17 The corresponding extensional relaxation time λ, break-up time tB, and the apparent modulus Gapp are depicted in Fig. 7d. All extracted parameters decrease with an increase in ASW concentration.Effects of seawater ions and in particular CaCl2
The effect of Ca2+ was investigated with respect to possible calcium-mediated ionic bridges and their implications on the dynamic properties of mucin. Fig. 8a depicts the measured (ergodic, see above) intermediate scattering functions of mucin solutions without and with added calcium at increasing concentrations: 0.005, 0.01 (natural), and 0.1 M of CaCl2. Measurements in saline environments showed a more pronounced slow mode. The evidence for Ca2+–ion bonds is also shown in Fig. 8b at q > 0.02225 nm−1 by the decrease in the apparent diffusion coefficient for salt-free samples and samples with 0.1 M of CaCl2. The decrease of the diffusion coefficient is generally observed for all added salts as depicted in Fig. 8b (large q-values as indicated by the arrow) and suggests a network formation of mucins. In the absence of salt (empty triangles) the apparent diffusion coefficient Dapp has the highest values and drops with the addition of salts. The highest reduction is seen for a mixture of 3 M of NaCl and 0.01 M CaCl2 (blue triangle). The DLS measurements were performed at very long-time intervals (4500 s) for the salt-free samples while measurements in saline environments were performed at long-time intervals as well as in cumulated 20 time intervals of 100 s (Fig. S6–S8, ESI†) for a mucin solution with 0.1 M CaCl2. The cumulated measurements were performed to obtain good statistics and extract reliable information about the main (fast) relaxation process (Fig. S7 and S8, ESI†) while the slow mode (long relaxation time) is captured in the long-time interval measurement.Mucin network formation by calcium is further supported by extensional rheology (Fig. 9). CaBER measurements show that both, the extensional relaxation time λ and break-up times tB follow the same trend: both values rise (Region I) until a peak is observed at the natural calcium concentration of 0.01 M. Above this concentration, both times constants rapidly decrease and reach a plateau (Region II). In Region I vesicles open up mediated by calcium and release mucin18,19 until the natural concentration of 0.01 M CaCl2 is reached. Beyond the natural calcium concentration, we speculate that the network collapses because of the attractive interactions, which dominate within mucin molecules (Ca2+ bridges).
IV. Discussion
Vesicle and mucin structure
In this study the structure and dynamics of hagfish mucin were investigated. A particular focus was put on the effect of mucin concentration and ionic composition of the surrounding liquid. As shown in Fig. 2, mucin vesicles are disk-shaped with sizes in the range of roughly 0.4 μm (height) to 2 μm (width) to 3–4 μm (length). The vesicles open up in contact with water and release mucin macromolecules, which form hagfish mucus – the part of hagfish slime without protein threads. AFM images revealed that hagfish mucins are in the micrometers length-scale, which agrees well with the large size reported for other mucins. However, unlike human or pig mucin glycoproteins, hagfish mucins did not show the typically observed “ball-and-chain structure”.34,35 Their different molecular composition – 80% protein and 20% sugar residues in contrast to other mucins that are 20% protein and 80% sugar residues8 – could be a possible explanation for the absence of the ball-and-chain structure.Mucin concentration dependence
The presence of large molecules with substantial polydispersity was further implied by the occurrence of a slow relaxation process in the DLS measurements under various environmental conditions (Fig. 4a, 7a and 8a). Due to their large size, the apparent diffusion coefficient depends on the scattering wavevector (Fig. 4b), which is observed for all measured mucin concentrations (Fig. S2, ESI†). The intensity profiles I(q) scale with q−3, highlighting the presence of a wide spanning network. Based on this result, the internal dynamics of the system are probed. The apparent static correlation length ξ of about ∼100 nm is smaller than the hydrodynamic correlation length ξH (∼ 400 nm) extracted from the apparent diffusion coefficient (Fig. S9, ESI†). However, both ξH and ξ are nearly insensitive to mucin and salt concentrations (Fig. S10, ESI†). The small but albeit unambiguous difference between these two length scales is not surprising for such large objects: it reflects the measurement of a highly heterogeneous mucin network (see Fig. 3) and the associated statistical analysis of the probed signal.36 The assignment of these length scales to the mesh size of the formed mucin network is supported by the AFM image of mucin solution in Fig. 3. It shows a heterogeneous network with long filaments which are entangled and the mesh size is polydisperse but of the order of 100 nm. By increasing the mucin concentration (Fig. S9, ESI†), we note that (i) the internal structure remains similar, (ii) the characteristic size (ξ and ξH) is unchanged and (iii) the moduli extracted from extensional rheology (i.e. Gapp) are almost independent of concentration. This evidence suggests that as the concentration increases the network structure (mesh size and associated modulus) does not change, but instead the length of the filaments increases. This is a likely scenario as single mucin molecules are known to dimerize and subsequently polymerize via disulfide bond formation.35,37,38 This behavior suggests self-similarity for hagfish mucins, which was already postulated for whole hagfish slime by Chaudhary et al.33DLS measurements for mucins in Milli-Q water further showed that the diffusion coefficient is constant at lower mucin concentrations but decreases at a mucin concentration of about cmucin = 0.2 mg ml−1. In Fig. 5, three distinct regions highlight the dilute (I), the semidilute (II), and the entanglement regime (III). For non-charged polymers the dilute and the semidilute regime are well distinguished by the overlapping of macromolecules leading to a slowdown of the diffusion coefficient. In charged systems such as polyelectrolytes and thus also for hagfish mucin, ions prevent the overlap and penetration of macromolecules. Their ion-impaired configuration gives rise to a constant diffusion coefficient in an extended concentration regime (regime I and II in Fig. 5).39 For hagfish mucin, the apparent diffusion coefficient remains constant for three decades in concentration before decreasing at cmucin = 0.2 mg ml−1. The entanglement concentration of 0.2 mg ml−1 is about ten times higher than the natural concentration and thus ten time less economic for the hagfish to rely as defense system.11 Polymeric systems operating below their entanglement concentration generally do not show pronounced and characteristic macroscopic flow features, which however is the case with hagfish slime. This raises the question, if together with the protein thread network the mucin could start to show its characteristic rheological behavior below the entanglement concentration by using the thread network as anchor points and thus similarly reach an entanglement concentration, as already suggested by us before.40
Rheological signature of the mucin network
The shear rheological properties of hagfish mucin is shown in Fig. 6a. At a mucin concentration of cmucin = 3 mg ml−1 a plateau for the storage modulus G′ and a minimum of the loss modulus G′′ is observed, which is the signature of a physical network and can be attributed to entangled or interacting mucin molecules. The mucin solution exhibits terminal flow after roughly 21 hours, suggesting that it is highly viscoelastic albeit with low plateau modulus. The latter value is in a good agreement with those reported for other mucin systems.41 This is the first time that the viscoelastic network of hagfish mucin was measured in shear rheology, i.e. in the absence of the protein threads. It is considered that in natural hagfish slime the protein threads dominate the rheological signal, mainly contributing to the observed viscoelasticity (G′ ∼ 0.02 Pa at natural conditions of mucins and protein threads).10,33 However, in absence of protein threads and at mucin concentrations higher than naturally employed, the elastic modulus G′ can exceed 0.5 Pa (Fig. 6a) resulting in networks strengths 100 times higher that of natural hagfish slime including the protein threads. These results suggest that the mucin component also contribute to the viscoelasticity in natural hagfish slime and supports the idea that protein threads act as anchor points in natural hagfish slime.35 In Fig. 6b the capillary thinning of hagfish mucin at different concentrations in Milli-Q water is shown. The increase of mucin concentration slows down the capillary thinning process and leads to a longer elasto-capillary thinning regime. Relaxation times extracted from the CaBER measurements increased with mucin concentration (Fig. 6c). At higher concentrated mucin solutions (e.g. above cmucin = 0.1 mg ml−1) slower filament thinning and eventually no break-up but beads-on-a-string formation is observed (data not shown). Extensional and shear rheology yield similar values of the viscoelastic moduli.The role of ions present in artificial seawater (ASW)
The presence of salts, both monovalent (NaCl, KCl, etc.) and divalent (CaCl2, MgCl2, etc.) increase the electrostatic screening within mucins due to the high ionic strength environment. Since the natural environment corresponds to 100% seawater, ions affect both the apparent diffusion coefficient and the rheological response of the mucin. In Fig. 7a, the similar shape of the I(q) curves including the presence of slow modes are attributed to the homogenous ion distribution. From the apparent diffusion coefficient, we extract the hydrodynamic radius RH of 400 nm, which is similar to the size in the salt-free environment (Milli-Q water). By increasing the concentration of seawater, the initial thinning dynamics in CaBER experiments were accelerated due to the high ionic strength of the ASW (Fig. 7c). Consequently, the relaxation time λ, the apparent elastic modulus Gapp, and the break-up time tB showed a reduction when the concentration of seawater was increased (Fig. 7d), which was similarly shown for the dynamic viscosity of hagfish mucin.17 In extensional rheology, we propose that the electrostatic screening hinders a coil-to-stretch transition of the mucin polyelectrolytes under flow and therefore causes shorter relaxation times and break-up times with increasing ASW concentration. Coil-stretch transitions are well known in dilute solutions of linear high-molecular-weight polymers in extensional flows. If strain rates are above a critical value, polymers unravel from a loose spherical shape to an extended state, causing a spectacular increase in extensional resistance.42,43 Two conditions must be satisfied in order for polymers to be stretched: first, the strain rate must be large enough to stretch the polymer, meaning the strain rate must exceed the relaxation rate (1/λ) of the molecule. And second, the strain rate must be applied for a sufficient time for the polymer molecule to accumulate the strain.44,45 For hagfish slime defense, reduced relaxation and break-up times in ASW compared to dilutions of ASW would mean less defensive potential as the loss in slime viscoelasticity was suggested to prevent efficient gill clogging with would-be predators.40 Above 0.003 M CaCl2 (i.e. 30% ASW) all vesicles open due to calcium mediated vesicle swelling18 whereas below 0.1 M NaCl also all vesicles open due to hypo-osmolarity.17 With all dilutions of ASW all vesicles should always be open – triggered by calcium or hypo-osmolarity and thus the observed effect is unlikely to be caused by mucin concentration but rather by electrostatic screening effects.The particular role of calcium ions
Herr et al.18 showed that Ca2+–ions are important for mucin vesicle decondensation by activating Ca2+-mediated transporter proteins in the vesicle membrane, which facilitate water influx and swelling of mucin vesicles. The authors showed that in seawater strength osmolarity a calcium concentration of 0.003 M is sufficient to trigger the opening of all mucin vesicles. Although calcium promotes the opening of the vesicles, concentrations exceeding 0.01 M (being the natural concentration in the sea) dramatically weakened the extensional response as can be seen by the nearly vanishing relaxation times (Fig. 9, Region II). These measurements suggest a structural collapse of the mucin molecules, which is also reflected in the DLS signals (Fig. 8) because they exhibit pronounced slow modes. The reduced extensional signal and occurrence of slow modes in DLS measurements strongly support the suggestion that calcium causes an ionotropic gelation, as mucins knowingly form reversible network formation and create salt-bridges between their chains with calcium.46–48V. Conclusions
Hagfish slime consists of two main components, mucin-like glycoproteins and protein threads initially coiled up in skeins, which together form a highly dilute hydrogel. Their synergistic interplay in high ionic strength seawater allows for a sophisticated and properly working defense system. We investigated the dynamics and structure of the mucin hydrogel in the presence and absence of seawater ions. Once in contact with water the hagfish mucin vesicles swell and release their mucin macromolecules. AFM imaging revealed the nano-morphology of the mucin vesicles, showing their disk like oval shape and a rough surface, which was confirmed with electron microscopy. Furthermore, the structure and size of single mucin molecules could be shown, revealing their large size (15–50 μm) and providing a mesh size. Using DLS, also the hydrodynamic radius (RH ∼ 400 nm) of the mucin macromolecules was obtained for both Milli-Q and ionic environment. The scattering intensity for all mucin concentrations scaled with the scattering wave vector q with a power-law slope of – 3, suggesting that the internal structure of mucin is independent of concentration and therefore possesses self-similar features. The emerging picture calls for a heterogeneous network whose structure is virtually unchanged with increasing concentration while concomitantly its constituting filaments increase in length by means of self-assembly. The entanglement concentration of hagfish mucin was found to be 0.2 mg ml−1. This concentration is one order of magnitude higher than the natural amount of mucin in hagfish slime (0.02 mg ml−1). Small oscillatory shear rheology revealed pronounced viscoelastic properties at high concentrations (3 mg ml−1), showing that mucin alone achieved mechanical properties similar to those of natural hagfish slime (mucins and protein threads). As the current opinion is that the mucin part in hagfish slime does not contribute to viscoelasticity, this finding implies that viscoelasticity of the natural slime may not exclusively be governed by the protein threads. The presence of seawater ions did not alter significantly the hydrodynamic radius RH compared to Milli-Q water. However, the thinning dynamics in extensional rheometry experiments were accelerated and consequently the relaxation time λ, the apparent elastic modulus Gapp, and the break-up time tB were reduced due to the high ionic strength of the ASW. It is likely that electrostatic screening hinders a coil-to-stretch transition of the mucin in the extensional flow. Calcium ions were found to strongly mediate viscoelasticity at concentrations below the natural calcium concentration in seawater (0.01 M). Calcium concentrations higher than 0.01 M lead to a collapse of structure of the mucin gel, which was manifested by the loss of the ability to form capillaries in extensional rheology as well as the occurrence of pronounced slow modes in DLS measurement. The observed slow mode in the intensity (I(q) curves) indicate a reduced dynamic of the topologically constrained mucin molecules, most likely caused by their polyelectrolyte character and their interaction with calcium ions. The reduced extensional signal and occurrence of slow modes in DLS measurements strongly support the suggestion that calcium leads to an ionotropic gelation of hagfish mucin. Future work, especially at higher mucin concentrations and mucus formulations will help further exploring the properties of this fascinating class of materials. In this respect, the eventual non-ergodicity and the possible emergence of surface layers during measurements are important challenges to address.Conflicts of interest
There are no conflicts to declare.Acknowledgements
LJB and PF acknowledge funding from ETH Zürich (Grant ETH-1914-1).References
- T. J. Parker and W. A. Haswell, A textbook of zoology, Macmillan, 1910 Search PubMed.
- V. Zintzen, C. D. Roberts, M. J. Anderson, A. L. Stewart, C. D. Struthers and E. S. Harvey, Hagfish predatory behaviour and slime defence mechanism, Sci. Rep., 2011, 1, 131 CrossRef CAS PubMed.
- J. Lim, D. S. Fudge, N. Levy and J. M. Gosline, Hagfish slime ecomechanics: testing the gill-clogging hypothesis, J. Exp. Biol., 2006, 209, 702–710 CrossRef PubMed.
- S. W. Downing, R. H. Spitzer, W. L. Salo, J. S. Downing, L. J. Saidel and E. A. Koch, Threads in the hagfish slime gland thread cells: organization, biochemical features, and length, Science, 1981, 212, 326–328 CrossRef CAS PubMed.
- B. Fernholm, Thread Cells from the Slime Glands of Hagfish (Myxinidae), Acta Zool., 1981, 62, 137–145 CrossRef.
- L. Böcker, P. A. Rühs, L. Böni, P. Fischer and S. Kuster, Fiber-Enforced Hydrogels: Hagfish Slime Stabilized with Biopolymers including κ-Carrageenan, ACS Biomater. Sci. Eng., 2016, 2, 90–95 CrossRef.
- L. Böni, P. A. Rühs, E. J. Windhab, P. Fischer and S. Kuster, Gelation of Soy Milk with Hagfish Exudate Creates a Flocculated and Fibrous Emulsion- and Particle Gel, PLoS One, 2016, 11, e0147022 CrossRef PubMed.
- W. L. Salo, S. W. Downing, W. A. Lidinsky, W. H. Gallagher, R. H. Spitzer and E. A. Koch, Fractionation of hagfish slime gland secretions: partial characterization of the mucous vesicle fraction, Prep. Biochem., 1983, 13, 103–135 CrossRef CAS PubMed.
- J. E. Herr, T. M. Winegard, M. J. O'Donnell, P. H. Yancey and D. S. Fudge, Stabilization and swelling of hagfish slime mucin vesicles, J. Exp. Biol., 2010, 213, 1092–1099 CrossRef CAS PubMed.
- R. H. Ewoldt, T. M. Winegard and D. S. Fudge, Non-linear viscoelasticity of hagfish slime, Int. J. Non Linear Mech., 2011, 46, 627–636 CrossRef.
- D. S. Fudge, N. Levy, S. Chiu and J. M. Gosline, Composition, morphology and mechanics of hagfish slime, J. Exp. Biol., 2005, 208, 4613–4625 CrossRef CAS PubMed.
- D. R. Kester, I. W. Duedall, D. N. Connors and R. M. Pytkowicz, Preparation Of Artificial Seawater, Limnol. Oceanogr., 1967, 12, 176–179 CrossRef CAS.
- L. J. Böni, R. Zurflüh, M. Widmer, P. Fischer, E. J. Windhab, P. A. Rühs and S. Kuster, Hagfish slime exudate stabilization and its effect on slime formation and functionality, Biol. Open, 2017, 6, 1115–1122 CrossRef PubMed.
- S. W. Downing, W. L. Salo, R. H. Spitzer and E. A. Koch, The hagfish slime gland: a model system for studying the biology of mucus, Science, 1981, 214, 1143–1145 CrossRef CAS PubMed.
- D. L. Luchtel, A. W. Martin and I. Deyrup-Olsen, Ultrastructure and permeability characteristics of the membranes of mucous granules of the hagfish, Tissue Cell, 1991, 23, 939–948 CrossRef CAS PubMed.
- M. A. Bernards Jr., I. Oke, A. Heyland and D. S. Fudge, Spontaneous unraveling of hagfish slime thread skeins is mediated by a seawater-soluble protein adhesive, J. Exp. Biol., 2014, 217, 1263–1268 CrossRef PubMed.
- L. J. Böni, R. Zurflüh, M. E. Baumgartner, E. J. Windhab, P. Fischer, S. Kuster and P. A. Rühs, Effect of ionic strength and seawater cations on hagfish slime formation, Sci. Rep., 2018, 8, 9867 CrossRef PubMed.
- J. E. Herr, A. M. Clifford, G. G. Goss and D. S. Fudge, Defensive slime formation in Pacific hagfish requires Ca2 – and aquaporin-mediated swelling of released mucin vesicles, J. Exp. Biol., 2014, 217, 2288–2296 CrossRef PubMed.
- M. A. Bernards Jr., S. Schorno, E. McKenzie, T. M. Winegard, I. Oke, D. Plachetzki and D. S. Fudge, Unraveling inter-species differences in hagfish slime skein deployment, J. Exp. Biol., 2018, 221, jeb176925 CrossRef PubMed.
- L. J. Böni, A. Sanchez-Ferrer, M. Widmer, M. D. Biviano, R. Mezzenga, E. J. Windhab, R. R. Dagastine and P. Fischer, Structure and Nanomechanics of Dry and Hydrated Intermediate Filament Films and Fibers Produced from Hagfish Slime Fibers, ACS Appl. Mater. Interfaces, 2018, 10, 40460–40473 CrossRef PubMed.
- P. Verdugo, I. Deyrup-Olsen, M. Aitken, M. Villalon and D. Johnson, Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding, J. Dent. Res., 1987, 66, 506–508 CrossRef CAS PubMed.
- S. H. Ching, N. Bansal and B. Bhandari, Alginate gel particles–A review of production techniques and physical properties, Crit. Rev. Food Sci. Nutr., 2017, 57, 1133–1152 CrossRef CAS PubMed.
- H. Kleine-Brueggeney, G. K. Zorzi, T. Fecker, N. E. El Gueddari, B. M. Moerschbacher and F. M. Goycoolea, A rational approach towards the design of chitosan-based nanoparticles obtained by ionotropic gelation, Colloids Surf., B, 2015, 135, 99–108 CrossRef CAS PubMed.
- M. A. Patel, M. H. H. AbouGhaly, J. V. Schryer-Praga and K. Chadwick, The effect of ionotropic gelation residence time on alginate cross-linking and properties, Carbohydr. Polym., 2017, 155, 362–371 CrossRef CAS PubMed.
- V. Pillay, C. M. Dangor, T. Govender, K. R. Moopanar and N. Hurbans, Ionotropic gelation: Encapsulation of indomethacin in calcium alginate gel discs, J. Microencapsulation, 1998, 15, 215–226 CrossRef CAS PubMed.
- J. Marakis, K. Wunderlich, M. Klapper, D. Vlassopoulos, G. Fytas and K. Müllen, Strong Physical Hydrogels from Fibrillar Supramolecular Assemblies of Poly(ethylene glycol) Functionalized Hexaphenylbenzenes, Macromolecules, 2016, 49, 3516–3525 CrossRef CAS.
- M. Shibayama, Y. Fujikawa and S. Nomura, Dynamic Light Scattering Study of Poly(N-isopropylacrylamide-co-acrylic acid) Gels, Macromolecules, 1996, 29, 6535–6540 CrossRef CAS.
- D. K. Carpenter, Dynamic Light Scattering with Applications to Chemistry, Biology, and Physics (Berne, Bruce J.; Pecora, Robert), J. Chem. Educ., 1977, 54, A430 CrossRef.
- A. G. Mailer, P. S. Clegg and P. N. Pusey, Particle sizing by dynamic light scattering: non-linear cumulant analysis, J. Phys.: Condens. Matter, 2015, 27, 145102 CrossRef PubMed.
- S. L. Anna and G. H. McKinley, Elasto-capillary thinning and breakup of model elastic l iquids, J. Rheol., 2001, 45, 115–138 CrossRef CAS.
- M. Gianneli, R. F. Roskamp, U. Jonas, B. Loppinet, G. Fytas and W. Knoll, Dynamics of swollen gel layers anchored to solid surfaces, Soft Matter, 2008, 4, 1443–1447 RSC.
- A.-M. Philippe, L. Cipelletti and D. Larobina, Mucus as an Arrested Phase Separation Gel, Macromolecules, 2017, 50, 8221–8230 CrossRef CAS.
- G. Chaudhary, D. S. Fudge, B. Macias-Rodriguez and R. H. Ewoldt, Concentration-independent mechanics and structure of hagfish slime, Acta Biomater., 2018, 79, 123–134 CrossRef CAS PubMed.
- R. Bansil, E. Stanley and J. T. Lamont, Mucin Biophysics, Annu. Rev. Physiol., 1995, 57, 635–657 CrossRef CAS PubMed.
- R. Bansil and B. S. Turner, Mucin structure, aggregation, physiological functions and biomedical applications, Curr. Opin. Colloid Interface Sci., 2006, 11, 164–170 CrossRef CAS.
- A. Aggeli, G. Fytas, D. Vlassopoulos, T. C. McLeish, P. J. Mawer and N. Boden, Structure and dynamics of self-assembling beta-sheet peptide tapes by dynamic light scattering, Biomacromolecules, 2001, 2, 378–388 CrossRef CAS PubMed.
- R. Bansil, J. P. Celli, J. M. Hardcastle and B. S. Turner, The Influence of Mucus Microstructure and Rheology in Helicobacter pylori Infection, Front. Immunol., 2013, 4, 310 Search PubMed.
- J. K. Sheehan, S. Kirkham, M. Howard, P. Woodman, S. Kutay, C. Brazeau, J. Buckley and D. J. Thornton, Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin, J. Biol. Chem., 2004, 279, 15698–15705 CrossRef CAS PubMed.
- R. H. Colby, Structure and linear viscoelasticity of flexible polymer solutions: comparison of polyelectrolyte and neutral polymer solutions, Rheol. Acta, 2010, 49, 425–442 CrossRef CAS.
- L. Böni, P. Fischer, L. Böcker, S. Kuster and P. A. Rühs, Hagfish slime and mucin flow properties and their implications for defense, Sci. Rep., 2016, 6, 30371 CrossRef PubMed.
- S. K. Lai, Y.-Y. Wang, D. Wirtz and J. Hanes, Micro- and macrorheology of mucus, Adv. Drug Delivery Rev., 2009, 61, 86–100 CrossRef CAS PubMed.
- P. P. Bhat, S. Appathurai, M. T. Harris, M. Pasquali, G. H. McKinley and O. A. Basaran, Formation of beads-on-a-string structures during break-up of viscoelastic filaments, Nat. Phys., 2010, 6, 625–631 Search PubMed.
- R. G. Larson and J. J. Magda, Coil-stretch transitions in mixed shear and extensional flows of dilute polymer solutions, Macromolecules, 1989, 22, 3004–3010 CrossRef CAS.
- P. G. D. Gennes and P. G. De Gennes, Coil-stretch transition of dilute flexible polymers under ultrahigh velocity gradients, J. Chem. Phys., 1974, 60, 5030–5042 CrossRef.
- E. J. Hinch, Mechanical models of dilute polymer solutions in strong flows, Phys. Fluids, 1977, 20, S22 CrossRef CAS.
- J. F. Forstner and G. G. Forstner, Calcium binding to intestinal goblet cell mucin, Biochim. Biophys. Acta, 1975, 386, 283–292 CrossRef CAS.
- B. D. E. Raynal, T. E. Hardingham, J. K. Sheehan and D. J. Thornton, Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus, J. Biol. Chem., 2003, 278, 28703–28710 CrossRef CAS PubMed.
- C. Ridley, N. Kouvatsos, B. D. Raynal, M. Howard, R. F. Collins, J.-L. Desseyn, T. A. Jowitt, C. Baldock, C. W. Davis, T. E. Hardingham and D. J. Thornton, Assembly of the respiratory mucin MUC5B: a new model for a gel-forming mucin, J. Biol. Chem., 2014, 289, 16409–16420 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sm00971j |
| This journal is © The Royal Society of Chemistry 2019 |






