Conversion of a surfactant-based microemulsion to a surfactant-free microemulsion by CO2†
Abstract
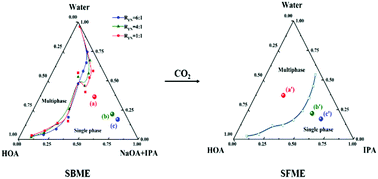
A microemulsion with a CO2 response was prepared by mixing the surfactant sodium oleate (NaOA), the co-surfactant isopropyl alcohol (IPA), oil phase oleic acid (HOA) and water. This surfactant-based microemulsion (SBME) shows a CO2 responsive behavior, and the introduction of CO2 can breakdown the microemulsion. Through the research in this paper, it is found that the content of IPA has a direct impact on the CO2 response behavior of SBME. It was found that the lower the IPA content (22.73 wt%), the more obvious the CO2 response behavior of SBME. Conversely, when the concentration of IPA is high (54.05 wt% and 63.83 wt%), the introduction of CO2 does not directly lead to the demulsification of the microemulsion. NaOA can be converted to HOA under the action of CO2, which is why SBME shows CO2 response behavior. By comparing the effects of CO2 on the (pseudo-)ternary phase diagrams of SBME and surfactant-free microemulsion (SFME), we found evidence that SBME shows different CO2 response behaviors. When CO2 was bubbled into the SBME system with a low IPA content, IPA cannot stabilize the excessive HOA and water in the system and eventually break the microemulsion. The situation is different when CO2 is applied to the SBME system with a high IPA content. IPA as an amphiphilic solvent can stabilize the HOA and water in the system to form SFME. In this process, SBME can be demulsified (low IPA content) or can be converted to SFME (high IPA content) in the presence of CO2.


 Please wait while we load your content...
Please wait while we load your content...