Polyelectrolyte–micelle coacervates: intrapolymer-dominant vs. interpolymer-dominant association, solute uptake and rheological properties†
Abstract
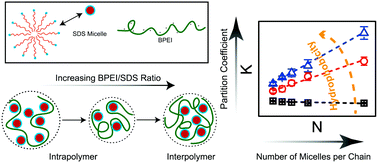
The effects of polyelectrolyte charge density, polyelectrolyte-to-surfactant ratio, and micelle species on coacervation were studied by turbidity, dynamic light scattering, and zeta potential measurements to examine the coacervation of the weak polyelectrolyte branched polyethylenimine (BPEI) and oppositely charged sodium dodecyl sulfate (SDS) micelles as well as BPEI and mixed micelles composed of SDS and poly(ethylene glycol) 4-nonylphenyl 3-sulfopropyl ether potassium salt (PENS). The results of dynamic light scattering and zeta potential measurements are discussed in terms of pH and BPEI-to-surfactant ratio. An intrapolymer-dominant to interpolymer-dominant association model for the BPEI–micelle coacervates was proposed based on the variation of size and zeta potential of coacervate particles by their BPEI-to-surfactant ratio. The partition coefficient of solutes into BPEI–micelle coacervates was determined using UV-vis measurements as a function of pH, BPEI-to-surfactant ratio, and mixed micelle composition. Both the hydrophobicity of solutes and micelles, as well as the association mode of coacervates, impact the solute uptake efficiency. Dynamic rheological measurements on the coacervates suggest that the rheological properties of the complex coacervates are impacted by the association mode of the coacervates as well as the charge density on BPEI chains during coacervation.


 Please wait while we load your content...
Please wait while we load your content...