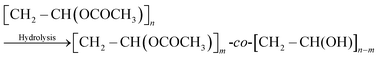The role of polymer–solvent interactions in polyvinyl-alcohol dispersions of multi-wall carbon nanotubes: from coagulant to dispersant†
Efrat
Ziv‡
a,
David
Attia‡
a,
Gleb
Vasilyev
b,
Orit
Mendelson
c,
Eyal
Zussman
 b and
Rachel
Yerushalmi-Rozen
b and
Rachel
Yerushalmi-Rozen
 *ad
*ad
aDepartment of Chemical Engineering, Ben-Gurion University of the Negev, 84105 Beer-Sheva, Israel. E-mail: rachely@bgu.ac.il
bNanoEngineering Group, Faculty of Mechanical Engineering, Technion – Israel Institute of Technology, 32000 Haifa, Israel
cDepartment of Chemistry, Nuclear Research Center-Negev, Beer-Sheva, Israel
dThe Ilse Katz Institute for Nanoscience and Technology, Ben-Gurion University of the Negev, 84105 Beer-Sheva, Israel
First published on 5th November 2018
Abstract
Dispersion of carbon nanotubes in solutions of polyvinyl-alcohol is required for solution casting of composite materials with improved interfacial adhesion where chains adsorbed on the nanotubes serve in the dual role of dispersant and compatible “connector” to the polyvinyl-alcohol matrix. Yet polyvinyl-alcohol is known to induce coagulation of nanotubes in aqueous solutions and thus far, it has not been used for dispersing pristine nanotubes. Here, we report that non-fully hydrolyzed (80–90%) polyvinyl-alcohol can be used for the preparation of stable, surfactant-free, dispersions of multi-wall carbon nanotubes in ethanol–water mixtures (of at least 50 vol% ethanol). Cryo-TEM imaging and rheological measurements of stable, long-lived dispersions reveal the formation of random networks of suspended tubes, with an averaged mesh size of ∼500 nm, indicating that the individual tubes do not aggregate or coagulate. We hypothesize that the polyvinyl-acetate sequences found in non-fully hydrolyzed polymers swell in the presence of ethanol, leading to the formation of a long-ranged steric (entropic) repulsion among polymer-decorated nanotubes. The unexpected role of the polyvinyl-acetate sequences along with a detailed dispersion mechanism are described.
Introduction
Poly-vinyl alcohol (PVA) is a biodegradable polymer with low toxicity and good bioadhesive characteristics. With a global production of more than 650![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year,1 the polymer is used for adhesives, fibers and textiles, packaging, pharmaceutical and biomedical applications. PVA is produced via polymerization of vinyl acetate to poly(vinyl acetate) (PVAc) followed by hydrolysis of PVAc to PVA. The degree of hydrolysis determines the fraction of the remaining (hydrophobic) acetate groups in the resulting polymer. Thus, the polymer may be regarded as an amphiphilic copolymer of PVA and PVAc. For a given molecular weight, the solubility, self-assembly and surface activity in aqueous and organic solvents are dominated by the degree of hydrolysis.2 Typical values range from 80 wt% hydrolysis (considered low) to 99 wt%.
000 tons per year,1 the polymer is used for adhesives, fibers and textiles, packaging, pharmaceutical and biomedical applications. PVA is produced via polymerization of vinyl acetate to poly(vinyl acetate) (PVAc) followed by hydrolysis of PVAc to PVA. The degree of hydrolysis determines the fraction of the remaining (hydrophobic) acetate groups in the resulting polymer. Thus, the polymer may be regarded as an amphiphilic copolymer of PVA and PVAc. For a given molecular weight, the solubility, self-assembly and surface activity in aqueous and organic solvents are dominated by the degree of hydrolysis.2 Typical values range from 80 wt% hydrolysis (considered low) to 99 wt%.
Sketch 1
The distribution of hydrolyzed monomers within the chain is mostly non-random and a few studies have quantified a degree of blockiness (intramolecular distribution of the comonomers) that depends on the details of the synthesis.3,4
It is well known that low concentrations of carbonaceous nanostructures, such as carbon nanotubes (CNTs), can improve the mechanical, thermal and electrical properties of PVA composites and hydrogels.5–7 Two of the conditions necessary for a significant improvement of the physical properties in CNT-reinforced polymeric matrices (and hydrogels) are a good dispersion of individual CNTs within the matrix and good interfacial adhesion between the pristine CNTs and the polymeric molecules.8 Yet, CNTs are difficult to disperse due to the large cohesion energy among the tubes.8 A simple non-destructive strategy for the dispersion of CNTs in a liquid carrier relies on sonication-assisted exfoliation followed by physical adsorption of solvated surfactants or polymers onto the CNTs. Good matching between the composition and structure of the dispersing agent and the target matrix leads to high interfacial adhesion.9
In particular, when a matrix-compatible polymer is used as the dispersing agent, the adsorbed molecules act as “anchors” and transfer stress across the CNTs–matrix interface.10,11 As PVA is mainly processed via wet spinning from aqueous solutions, dispersion of CNTs in aqueous solutions of PVA would be highly beneficial. However, previous reports suggest that PVA cannot be used as a dispersing agent for non-functionalized CNTs as the polymer induces aggregation and coagulation of the pristine CNTs in aqueous solutions.12,13 Indeed, most of the reported CNTs–PVA composites are prepared either by functionalization of the CNTs,14 or with the aid of a dispersing agent that is not compatible with the PVA matrix. In these composites, the interfacial contact is formed between the (incompatible) molecular layer coating the CNTs and the PVA matrix, most often leading to a less-than optimal interfacial adhesion14 unless the dispersing agent is removed at some stage of the processes. The challenge is thus to formulate a stable (or long-lived) dispersion of CNTs using PVA as the dispersing agent, and elucidate the nano-structure of the dispersion and the dispersion mechanism.
In the study reported here, we found that in accordance with the literature, PVA of different molecular weights and degrees of hydrolysis does not disperse different types of multi-wall carbon nanotubes (MWNTs) in water. However, stable dispersions of MWNTs could be obtained via mild sonication of MWNT powders in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions of PVA of low-to-medium degree of hydrolysis (80 to 90%) with an EtOH volume fraction of 50 vol%, but not when the degree of hydrolysis of the PVA is higher (98%). The dispersion mechanism of CNTs in polymer solutions relies on polymer adsorption onto the CNTs followed by the onset of steric repulsion among polymer-decorated CNTs.6 Our studies indicate that while PVA adsorption onto MWNTs already takes place in aqueous solutions, addition of EtOH, a non-solvent for PVA15 and a good solvent for PVAc,15 triggers longer range entropic repulsion among polymer-decorated MWNTs overriding the short-range attractive potential of the MWNTs and thus preventing re-aggregation of the nanotubes. The well-dispersed state of MWNTs in 50 vol% EtOH
water solutions of PVA of low-to-medium degree of hydrolysis (80 to 90%) with an EtOH volume fraction of 50 vol%, but not when the degree of hydrolysis of the PVA is higher (98%). The dispersion mechanism of CNTs in polymer solutions relies on polymer adsorption onto the CNTs followed by the onset of steric repulsion among polymer-decorated CNTs.6 Our studies indicate that while PVA adsorption onto MWNTs already takes place in aqueous solutions, addition of EtOH, a non-solvent for PVA15 and a good solvent for PVAc,15 triggers longer range entropic repulsion among polymer-decorated MWNTs overriding the short-range attractive potential of the MWNTs and thus preventing re-aggregation of the nanotubes. The well-dispersed state of MWNTs in 50 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water PVA solutions is demonstrated via cryo-TEM images and rheological measurements. At a lower EtOH content, cryo-TEM images show MWNT aggregation and the flow behavior of the dispersions is Newtonian, probably as re-aggregation of the suspended MWNTs reduces the concentration of the suspended MWNTs to below the percolation threshold.
water PVA solutions is demonstrated via cryo-TEM images and rheological measurements. At a lower EtOH content, cryo-TEM images show MWNT aggregation and the flow behavior of the dispersions is Newtonian, probably as re-aggregation of the suspended MWNTs reduces the concentration of the suspended MWNTs to below the percolation threshold.
Experimental section
Materials
The polymers are denoted by their molecular weights and degree of hydrolysis. For example, PVA Mw 9000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 with a degree of hydrolysis of 80%, [CH2–CH(OCOCH3)]38-co-[CH2–CH(OH)]152, is denoted PVA10–80.
000 g mol−1 with a degree of hydrolysis of 80%, [CH2–CH(OCOCH3)]38-co-[CH2–CH(OH)]152, is denoted PVA10–80.
The different samples of MWNTs were prepared via catalytic CVD and used as received.
EtOH 99.9% was purchased from Sigma Aldrich Israel. Millipore water was used (resistance of 18.2 MΩ cm).
Preparation of solutions
Polymer solutions were prepared by dissolving the polymer powders in the desired solvent (water or water–ethanol mixtures) at room temperature (22–24 °C) or under heating (50 °C, high molecular weights of PVA of a high degree of hydrolysis) to the final concentrations. Solutions were stirred for 2–4 hours.Preparation of dispersions
Dispersions were prepared by addition of the solutions to powders of carbonaceous materials (CNTs, CB) (1–2 mg ml−1) followed by sonication under mild conditions (50 W, 43 kHz) for 1–2 hours. Dispersions that appeared to be stable were centrifuged (at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 20 min), and the supernatant was decanted and stored at room temperature.
000g for 20 min), and the supernatant was decanted and stored at room temperature.
Cryogenic transmission electron microscopy (cryo-TEM)
The method enables direct imaging of a nanostructure in aqueous solutions. Samples were prepared by applying a 3 μl drop to a glow discharge treated TEM grid (300 mesh Cu Lacey substrate, Ted Pella, Ltd). The excess liquid was blotted and the specimen was vitrified by rapid plunging into liquid ethane pre-cooled by liquid nitrogen using a vitrification robot system (Vitrobot mark IV, FEI). The rapid cooling results in physical fixation of the liquid state and allows examination of the dispersion in the high vacuum of the electron microscope at cryogenic temperature, which prevents the formation of either cubic or hexagonal ice. The vitrified samples were examined at −177 °C using an FEI Tecnai 12 G2 TWIN TEM operated at 120 kV and equipped with a Gatan model 626 cold stage. The images were recorded by using a 4k × 4k FEI Eagle CCD camera in low dose mode. TIA (Tecnai Imaging & Analysis) software was used to record the images.Surface tension measurements
Measurements were carried out using the Du Noüy ring method (KSV Sigma70 tensiometer) at room temperature. Samples were prepared by dilution of a stock solution to the final concentration.Rheological measurements
A Discovery DHR-2 rotational rheometer (TA Instruments, USA) was used to characterize the rheological properties of solutions under steady-state shear flow. Parallel-plate geometry, with a diameter of 40 mm and a gap of 0.35 mm, was applied. All the measurements were performed at 25 °C.Results and discussion
Dispersion of MWNTs in aqueous PVA solutions
In accordance with the published literature,1–4,10–12 we found that MWNTs could not be dispersed in aqueous solutions of PVA. PVA polymers of different molecular weights and degrees of hydration were tested. Unsuccessful attempts to disperse MWNTs in aqueous solutions of PVA are summarized in Table S1 of the ESI.† Similar solutions successfully dispersed carbon black (CB) (Fig. S1 and S2, ESI†).Dispersion of MWNTs in PVA solutions that contain EtOH
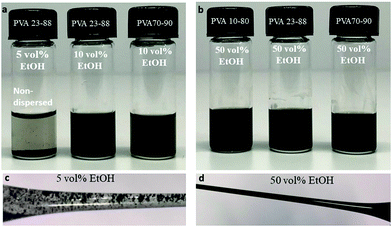
We found that dispersions of MWNTs could be formed in PVA solutions (1 and 5 wt%) that contained above 10 vol% EtOH (MWNTs-NTX4) and above 30 vol% EtOH (MWNTs-INP). The dispersions were incubated at room temperature for 2–4 weeks, and designated as “stable” if macroscopic agglomeration and phase separation were not observed. The two types of MWNTs used in this study are of similar dimensions (but different manufacturers, see Table 2). In accordance with common knowledge,16 we observed that the solution behavior of the two MWNT samples differ in the details but exhibit similar overall trends.Visual inspection (Fig. 1) allows the identification of unsuccessful attempts to disperse MWNTs and macroscopic aggregation. Dispersions (Fig. 1b and d) that have the appearance of black, ink-like liquid were further tested using rheology and cryo-TEM.
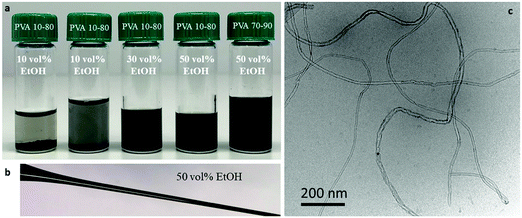
Cryo-TEM images (Fig. 2) clearly show that dispersions of MWNTs-NTX4 in 50 vol% EtOH and partially hydrolyzed PVA comprise randomly suspended mostly individual tubes (Fig. 2b and c). Microscopic aggregation is observed in solutions of similar polymer concentrations and 10 vol% ethanol (Fig. 2a). As observed in Fig. 3, a higher concentration of PVA (5 wt%, Fig. 3a) is necessary for dispersing MWNTs-INP. Well dispersed individual tubes are observed in the cryo-TEM images of vitrified dispersions containing 50 vol% EtOH (Fig. 3c).
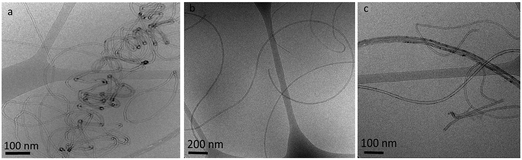 | ||
Fig. 2 Cryo-TEM images of vitrified dispersions of MWNTs-NTX4 (1 mg ml−1) in PVA solutions (PVA10–80, 1 wt%) in (a) EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 10 water 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol% and (b and c) EtOH 90 vol% and (b and c) EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 50 water 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol%. 50 vol%. | ||
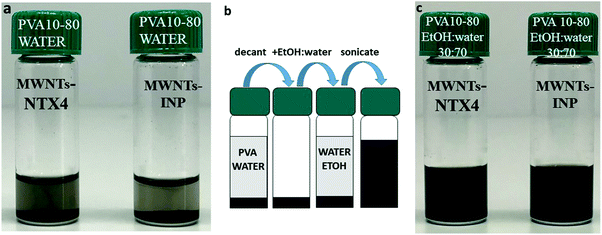
In a different set of experiments, powders of MWNTs (both MWNTs-NTX4 and MWNTs-INP) were sonicated in an aqueous solution of PVA10–80 for 2 h. Shortly after the sonication, the dispersions aggregated (see Fig. 4a). In a second step, EtOH was added to the aqueous polymer solution (to a final EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture of 50
water mixture of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol%), and the resulting solution was sonicated for 30 min. As can be clearly seen in Fig. 4b, the addition of EtOH enabled the formation of a stable dispersion that did not aggregate or coagulate for many weeks. We note here that prolonged sonication (up to 4 h) in aqueous PVA solutions did not lead to dispersion of the MWNTs (in the absence of EtOH), and nor did prolonged sonication in EtOH
50 vol%), and the resulting solution was sonicated for 30 min. As can be clearly seen in Fig. 4b, the addition of EtOH enabled the formation of a stable dispersion that did not aggregate or coagulate for many weeks. We note here that prolonged sonication (up to 4 h) in aqueous PVA solutions did not lead to dispersion of the MWNTs (in the absence of EtOH), and nor did prolonged sonication in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions, in the absence of PVA (see Fig. S3 in the ESI†).
water solutions, in the absence of PVA (see Fig. S3 in the ESI†).
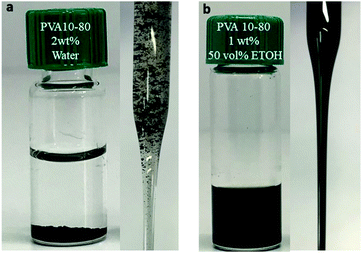 | ||
| Fig. 4 Dispersion of MWNTs-INP (1 mg ml−1) in solutions of PVA10–80 (a) 2 wt% polymer in water, after sonication and (b) after addition of EtOH to a final volume of 50% and sonication. | ||
The dispersion mechanism acting in this system is expected to rely on steric repulsion among polymer-decorated CNTs. Thus, both adsorption of the polymer onto the CNTs from solution and the onset of steric repulsion among polymer-decorated CNTs are necessary for the formation of a stable dispersion16,17 (see below). An intriguing question is what is the role of EtOH in the dispersion mechanism? It would be reasonable to assume that as EtOH is a non-solvent for PVA, it could enhance the surface activity of the polymer by reducing its solubility in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures, and thus promote polymer adsorption onto the hydrophobic MWNTs. While the MWNTs–solution interface is hard to access, we expect that a similar reasoning would promote PVA adsorption from EtOH
water mixtures, and thus promote polymer adsorption onto the hydrophobic MWNTs. While the MWNTs–solution interface is hard to access, we expect that a similar reasoning would promote PVA adsorption from EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions at the solution–air (hydrophobic) interface. To test this assumption, we measured the surface tension of PVA10–80 in water and in EtOH–water mixtures (Fig. 5). We used the Gibbs adsorption equation to calculate the surface excess (Γ) of PVA:
water solutions at the solution–air (hydrophobic) interface. To test this assumption, we measured the surface tension of PVA10–80 in water and in EtOH–water mixtures (Fig. 5). We used the Gibbs adsorption equation to calculate the surface excess (Γ) of PVA: 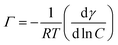 (R – gas constant, T – the absolute temperature, γ – surface tension, C – molar concentration). We observed a reduction in the PVA surface excess with the volume fraction of EtOH: in pure water, Γ = 2 × 10−6 mol m−2, in EtOH
(R – gas constant, T – the absolute temperature, γ – surface tension, C – molar concentration). We observed a reduction in the PVA surface excess with the volume fraction of EtOH: in pure water, Γ = 2 × 10−6 mol m−2, in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 10
water 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol%, Γ = 5 × 10−7 mol m−2. Similar measurements at 50 vol% EtOH indicate that the polymer does not adsorb at the air–water interface (Γ = 0 mol m−2). Note that while a reduction of surface tension due to the addition of EtOH is trivial, here, we observe a reduction of the surface excess of the solvated polymer (in the solvent mixture), indicating that EtOH reduces the surface activity of the polymer. The latter is equivalent to a surprising observation (supported by rheological measurements, indicating that the overlap concentration of PVA is similar in water and in water–ethanol mixtures, not shown) of an overall improved solubility of the PVA–PVAc copolymer in the EtOH
90 vol%, Γ = 5 × 10−7 mol m−2. Similar measurements at 50 vol% EtOH indicate that the polymer does not adsorb at the air–water interface (Γ = 0 mol m−2). Note that while a reduction of surface tension due to the addition of EtOH is trivial, here, we observe a reduction of the surface excess of the solvated polymer (in the solvent mixture), indicating that EtOH reduces the surface activity of the polymer. The latter is equivalent to a surprising observation (supported by rheological measurements, indicating that the overlap concentration of PVA is similar in water and in water–ethanol mixtures, not shown) of an overall improved solubility of the PVA–PVAc copolymer in the EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture. Thus, the hypothesis that the presence of ethanol promotes PVA adsorption (at any interface) is indirectly falsified by the observation that the polymer is depleted from the air–water interface. Recalling that the polymers used in this study are amphiphilic copolymers comprising PVA and PVAc sequences, and that EtOH is a good solvent for PVAc, we further suggest that preferential swelling of PVAc segments adsorbed at the MWNT surface by ethanol may play a role in the dispersion mechanism.
water mixture. Thus, the hypothesis that the presence of ethanol promotes PVA adsorption (at any interface) is indirectly falsified by the observation that the polymer is depleted from the air–water interface. Recalling that the polymers used in this study are amphiphilic copolymers comprising PVA and PVAc sequences, and that EtOH is a good solvent for PVAc, we further suggest that preferential swelling of PVAc segments adsorbed at the MWNT surface by ethanol may play a role in the dispersion mechanism.
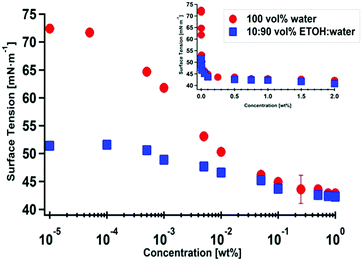 | ||
Fig. 5 Surface tension as a function of (log) polymer concentration, PVA10–80 in water and EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 10 water 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol%. The inset shows a larger concentration range on a linear scale. 90 vol%. The inset shows a larger concentration range on a linear scale. | ||
To test this assumption, we carried out two sets of experiments: (1) the solvent-exchange experiment presented in Fig. 6 and (2) dispersion experiments using PVA of a high degree of hydrolysis.
Powders of MWNTs (both MWNTs-NTX4 and MWNTs-INP) were sonicated in an aqueous solution of PVA for 2 h. Following sonication, the MWNTs aggregated and precipitated (see Fig. 6a). The solvent was decanted from above the precipitate and a (polymer free) EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture (30
water mixture (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 vol%) was added to the precipitate, re-sonicated for 120 min, and centrifuged. This process resulted in the formation of a black ink-like dispersion (Fig. 6c). As the EtOH
70 vol%) was added to the precipitate, re-sonicated for 120 min, and centrifuged. This process resulted in the formation of a black ink-like dispersion (Fig. 6c). As the EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture did not contain polymer, the only polymer in the system must have come from the first stage, suggesting that PVA adsorbed onto the MWNTs from water. It is well known that polymers do not desorb upon dilution and thus the results of this experiment suggest that while PVA chains adsorbed onto MWNTs from water, a dispersion only formed in the presence of EtOH, probably due to swelling of the PVAc segments of the adsorbed polymer and the consequential re-arrangement of the polymers at the CNT–solution interface.
water mixture did not contain polymer, the only polymer in the system must have come from the first stage, suggesting that PVA adsorbed onto the MWNTs from water. It is well known that polymers do not desorb upon dilution and thus the results of this experiment suggest that while PVA chains adsorbed onto MWNTs from water, a dispersion only formed in the presence of EtOH, probably due to swelling of the PVAc segments of the adsorbed polymer and the consequential re-arrangement of the polymers at the CNT–solution interface.
Solutions of PVA with a high degree of hydrolysis (98%, designated PVA 23–98, see Table 1) were investigated as well. EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water liquid mixtures (50
water liquid mixtures (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol%) were used for dissolution of PVA and MWNTs (both MWNTs-NTX4 and MWNTs-INP) and sonicated for 2 h. The MWNT powders were not dispersed, but rather aggregated at the bottom of the vial (see Fig. S4, ESI†). These observations indicate that the presence of a minimal fraction of PVAc is necessary for successful dispersion of the MWNTs in EtOH
50 vol%) were used for dissolution of PVA and MWNTs (both MWNTs-NTX4 and MWNTs-INP) and sonicated for 2 h. The MWNT powders were not dispersed, but rather aggregated at the bottom of the vial (see Fig. S4, ESI†). These observations indicate that the presence of a minimal fraction of PVAc is necessary for successful dispersion of the MWNTs in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions.
water solutions.
| Polymer | M w (g mol−1) | Degree of hydrolysis (%) | Designation |
|---|---|---|---|
| a Polydispersity index: 1.8–2.2. | |||
| PVA (80–90% degree of hydrolysis) | 9000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
80 | PVA10–80 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–23 000–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
87–88 | PVA23–88 | |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–70 000–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
87–90 | PVA70–90 | |
| PVA (97–98% degree of hydrolysis) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–23 000–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
98 | PVA23–98 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–85 000–85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
97 | PVA85–97 | |
| Batch | Manufacturer | Diameter and length | Purity (vol%) |
|---|---|---|---|
| MWNTs-NTX4 | Nanothinx, Greece | d: 10–20 nm | >90 |
| L: ≥10 μm | |||
| MWNTs-INP | Toulouse, ENSIACET | d: 10–20 nm | >95 |
| L: ≥10 μm | |||
| Carbon black | Cabot Corp. Canada | Spherical, 50–100 nm | |
TGA measurements of the dried dispersions were carried out as well (see the ESI† for details). The results indicate a weight ratio of about 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PVA to MWNTs-NTX4 in dispersions containing 50 vol% EtOH and lower ratios for the different combinations (Table S2, ESI†). The TGA data clearly show that only about 20 wt% of the initial CNT powder is dispersed. These results are consistent with previous studies.
1 PVA to MWNTs-NTX4 in dispersions containing 50 vol% EtOH and lower ratios for the different combinations (Table S2, ESI†). The TGA data clearly show that only about 20 wt% of the initial CNT powder is dispersed. These results are consistent with previous studies.
Rheological measurements of the dispersions were used to probe the dynamic behavior of the suspended nano-structures. In Fig. 7, we present the relative viscosity (the effective viscosity of the dispersion divided by the viscosity of the corresponding PVA solution) as a function of shear stress. Therefore, only the contribution of the suspended nanostructures is taken into account. It can be seen that the MWNTs-INP in a PVA solution of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol% EtOH
90 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water and CB in a PVA solution of 50
water and CB in a PVA solution of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% EtOH
50 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water do not contribute to the viscosity, thus the relative viscosity is near 1.0. In contrast, an increase of 2.7–3.2 times in the relative viscosity is observed in the region of low stress for both MWNT suspensions in PVA 50
water do not contribute to the viscosity, thus the relative viscosity is near 1.0. In contrast, an increase of 2.7–3.2 times in the relative viscosity is observed in the region of low stress for both MWNT suspensions in PVA 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% EtOH
50 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions, suggesting a strong contribution of the MWNTs to the rheological response of the dispersions.
water solutions, suggesting a strong contribution of the MWNTs to the rheological response of the dispersions.
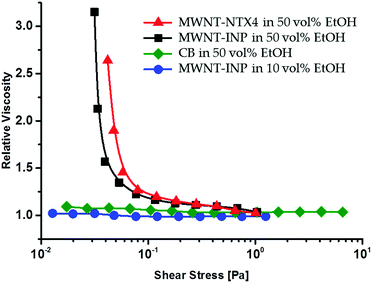 | ||
| Fig. 7 Relative viscosity vs. shear stress for MWNT and CB suspensions (2 mg ml−1 and 5 wt% PVA10–80). | ||
At the same time, the MWNTs do not contribute to the viscosity at high shear stress.
As was suggested by Du et al.,18 assembly of a transient network of nanotubes in polymeric dispersions leads to the appearance of a viscoelastic response. Furthermore, while in poor dispersions, discrete nanotube-rich domains (rather than a nanotube network) lead to a flow behavior of the polymer chains that is independent of the presence of the nanotubes, a yield behavior is expected (and observed) when the tubes are well dispersed and assemble into a transient network, as is the case in dispersions that contain 50 vol% EtOH. This yield behavior causes the appearance of a plateau region in the dynamic modulus vs. frequency curves in the region of low frequencies and characterizes properties of the structures formed by the transient network. Then, the mesh size of this network, that is, the distance between two neighbor cross-links (permanent or temporal), can be estimated when using the following equation:19
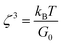 | (1) |
| B ∼ (φ − φc)n | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% EtOH
50 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water is equal to the determined yield stress, namely ∼0.03 Pa. Then, based on eqn (1), the averaged mesh size is ∼500 nm.
water is equal to the determined yield stress, namely ∼0.03 Pa. Then, based on eqn (1), the averaged mesh size is ∼500 nm.
The data presented so far indicate that dispersions of MWNTs can be formed in EtOH–water solutions of non-fully hydrolyzed PVA (degree of hydrolysis up to 90%). The minimal concentration of PVA depends on the MWNT sample: 1 wt% is required for MWNTs-NTX4 and 5 wt% for MWNTs-INP. When the EtOH fraction is 50 vol%, the dispersions are long-lived (up to weeks) and comprise non-aggregated, entangled networks of individual MWNTs that exhibit non-Newtonian flow behavior. At a lower volume fraction of EtOH, 10 vol% EtOH (MWNTs-NTX4) and 30 vol% EtOH (MWNTs-INP), rheological and cryo-TEM measurements indicate that aggregation of the MWNTs though macroscopic coagulation does not occur.
Polymer-assisted dispersion of CNTs in solutions relies on the onset of steric repulsion among polymer-decorated tubes so as to overcome the inter-tube potential. For MWNTs, the interaction between two parallel MWNTs may be described by the Girifalco potential.23 The latter is characterized by a steep attraction at a distance and depth that strongly depends on the tube diameter. The calculated contact energy24 is 138kBT/nm for MWNTs with d = 10 nm and 193kBT/nm for MWNTs with d = 20 nm (where kB is the Boltzmann constant and kBT is the thermal energy). The high cohesion energy prevents a spontaneous dispersion of MWNTs in liquid media. In typical sonication-assisted non-covalent methods for the dispersion of CNTs, the nanotubes are sonicated in the polymer solution, and the high energy provided by sonication leads to de-aggregation and temporary dispersion of the tubes.25 Polymer adsorption then takes place at the freshly exposed tube-walls and is followed by long-term rearrangement.26 In this scenario, two parameters affect the range of the steric repulsion among the nanostructures and thus the stability of the dispersion: the surface coverage of the polymer and the conformation of the adsorbed polymers. The solvent exchange experiment presented in Fig. 6 indicated that the PVA adsorbs onto the MWNT surface from water. Yet a dispersion is only formed in a mixed EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent. This is a somewhat surprising observation as water is a good solvent for non-fully hydrolyzed PVA in the low concentration regime relevant for this study. Under these conditions, one may assume that most of the adsorbed chains decorate the MWNTs in extended “loops”. However, the range of entropic repulsion induced by these loops is somewhat shorter than that required for stabilization of the MWNTs (Fig. 8a). Addition of EtOH, a non-solvent for PVA but a good solvent for the PVAc segments present in non-fully hydrolyzed PVA, leads to conformational re-arrangement at the (hydrophobic) MWNTs–solution interface. We speculate that the volume fraction of ethanol is somewhat higher at this interface, as is the volume fraction of PVAc segments. Conformational re-arrangement of the PVA-co-PVAc polymer in the EtOH–water mixture, and specifically swelling of the PVAc sequences, may increase the range of steric repulsion just enough to prevent aggregation of the polymer-decorated MWNTs (Fig. 8b). The effect of the EtOH fraction on the solvation of PVA is lower than may be expected when a poor solvent is added. As indicated by the surface excess values (Fig. 5) EtOH does not increase the surface excess of the polymer (Γ = 2 × 10−6 mol m−2 in water and Γ = 5 × 10−7 mol m−2 in 10
water solvent. This is a somewhat surprising observation as water is a good solvent for non-fully hydrolyzed PVA in the low concentration regime relevant for this study. Under these conditions, one may assume that most of the adsorbed chains decorate the MWNTs in extended “loops”. However, the range of entropic repulsion induced by these loops is somewhat shorter than that required for stabilization of the MWNTs (Fig. 8a). Addition of EtOH, a non-solvent for PVA but a good solvent for the PVAc segments present in non-fully hydrolyzed PVA, leads to conformational re-arrangement at the (hydrophobic) MWNTs–solution interface. We speculate that the volume fraction of ethanol is somewhat higher at this interface, as is the volume fraction of PVAc segments. Conformational re-arrangement of the PVA-co-PVAc polymer in the EtOH–water mixture, and specifically swelling of the PVAc sequences, may increase the range of steric repulsion just enough to prevent aggregation of the polymer-decorated MWNTs (Fig. 8b). The effect of the EtOH fraction on the solvation of PVA is lower than may be expected when a poor solvent is added. As indicated by the surface excess values (Fig. 5) EtOH does not increase the surface excess of the polymer (Γ = 2 × 10−6 mol m−2 in water and Γ = 5 × 10−7 mol m−2 in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 EtOH
90 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture). A similar behavior was reported for PVA hydrogels.27
water mixture). A similar behavior was reported for PVA hydrogels.27
As was discussed by us before, MWNTs interact via a short ranged attraction and are thus highly sensitive to variations in the long-range tail of the inter-particle potential.28,29 For a relatively weak potential barrier of about 5kBT/nm,16,17 an increase in the range of the entropic barrier from 2–3 nanometers to about 10 nanometers would be enough to prevent the tubes from approaching the short-ranged attractive region of the potential, and thus it would prevent re-aggregation and stabilize the dispersed tubes. Indeed, when PVA is not fully-hydrolyzed (and contains PVAc segments), addition of EtOH improves the dispersion probably due to re-arrangements of polymers adsorbed at the MWNTs–solution interface.
The results of the rheological measurements presented in Fig. 7 (and Fig. S4, ESI†) are in agreement with the proposed mechanism. MWNTs dispersed in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 vol% EtOH
90 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures do not contribute to the rheological response of the dispersion. They behave as Newtonian fluids where the viscosity is independent of shear stress in the full range of the applied stresses. This suggests that the suspended MWNTs do not form percolated structures, as the latter would result in a yield in the region of low stresses.30 In 50
water mixtures do not contribute to the rheological response of the dispersion. They behave as Newtonian fluids where the viscosity is independent of shear stress in the full range of the applied stresses. This suggests that the suspended MWNTs do not form percolated structures, as the latter would result in a yield in the region of low stresses.30 In 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% EtOH
50 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures, the rheological behavior is clearly different (Fig. 7): a sharp viscosity drop in the region of stresses of 0.04–0.08 Pa is detected for both MWNTs-INP and MWNTs-NTX4 dispersions. This viscosity drop is usually associated with the destruction of structures formed by suspended nanostructures. The values of the critical stress, the yield stress estimated using Casson model,31 are very close for both of the MWNTs and lie in the range of 0.02–0.04 Pa. Thus, although 10–30 vol% EtOH leads to the formation of black ink-like MWNT dispersions, the rheological response of the dispersions is consistent with mesoscopic re-aggregation leading to an effective number of suspended objects that is below the percolation threshold for MWNTs, and thus mesoscopic networks are not formed. Assuming that the dimensions of adsorbed PVA chains in the 50
water mixtures, the rheological behavior is clearly different (Fig. 7): a sharp viscosity drop in the region of stresses of 0.04–0.08 Pa is detected for both MWNTs-INP and MWNTs-NTX4 dispersions. This viscosity drop is usually associated with the destruction of structures formed by suspended nanostructures. The values of the critical stress, the yield stress estimated using Casson model,31 are very close for both of the MWNTs and lie in the range of 0.02–0.04 Pa. Thus, although 10–30 vol% EtOH leads to the formation of black ink-like MWNT dispersions, the rheological response of the dispersions is consistent with mesoscopic re-aggregation leading to an effective number of suspended objects that is below the percolation threshold for MWNTs, and thus mesoscopic networks are not formed. Assuming that the dimensions of adsorbed PVA chains in the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% EtOH
50 vol% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture are large enough to keep the dispersed MWNTs from re-aggregating, the dispersions would comprise mostly individual tubes and the effective fraction of the suspended nano-structures (MWNTs) would be above the (rheological) percolation threshold, causing self-assembly of mesoscopic networks, as observed in the measurements.
water mixture are large enough to keep the dispersed MWNTs from re-aggregating, the dispersions would comprise mostly individual tubes and the effective fraction of the suspended nano-structures (MWNTs) would be above the (rheological) percolation threshold, causing self-assembly of mesoscopic networks, as observed in the measurements.
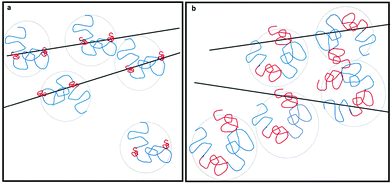
What about CB dispersions? Particle geometry and dimensions (here, the diameter) are found to play an important role: our observations show that CB is dispersed by PVA in water, and the dispersed CB aggregates (Fig. S1 and S2, ESI†) do not further coagulate. Yet the particles do not contribute to the viscosity of the dispersion and the flow behavior is fully Newtonian (Fig. S5, ESI†). While CB and MWNTs are carbonaceous nanostructures of similar composition, CB comprises colloidal particles with a single particle diameter in the range of 50–100 nm, with a typical aspect ratio (length/diameter) of 1, and the MWNT diameter ranges from 2–3 nm to about 10 nm, with an aspect ratio of 103. As was reported before by Szleifer et al.,16 detailed calculations show that when the diameter of the dispersed particle (CB) is much larger than the typical radius of the solvated polymer, adsorbed chains stretch in the direction normal to the surface while the conformation of chains adsorbed onto the MWNTs is hardly distorted, as compared to that of solvated chains (Fig. 9). As stretching of the chains increases the range of entropic repulsion, the PVA chains adsorbed onto CB from water form a steric barrier at a large enough range so as to prevent coagulation of the CB. Yet as the percolation threshold for the spherical CB particles is an order of magnitude higher than their concentration in the dispersions,32 they do not form a mesoscopic network and do not contribute significantly to the rheological behavior of the dispersion.
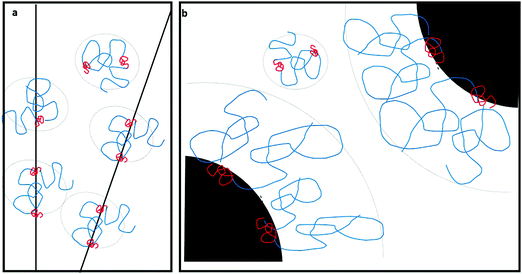 | ||
| Fig. 9 Schematic drawings presenting the interaction between polymer decorated nanostructures in water. The conformation of the adsorbed copolymer (PVA (blue)-co-PVAc (red)) on the (a) MWNTs is similar to that of the free polymer in solutions (b) while chains adsorbed onto CB stretch in the direction normal to the surface (following the calculations presented in ref. 15). | ||
Conclusions
Carbon nanotubes can be used for improving the mechanical, thermal and electrical properties of PVA composites and hydrogels.5–7 While co-processing of PVA and CNTs from aqueous solutions is expected to improve the interfacial adhesion at the polymer–CNT interface in the resulting composites,5,8,9 PVA is known to act as a coagulant in dispersions of CNTs. The findings presented here indicate that PVA can be used to prepare stable dispersions of individual MWNTs in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions, as long as the degree of hydration of the polymer is ≤90%. Experimental observations indicate that above a threshold concentration of EtOH (and PVA), long-lived dispersions are formed. The dispersions comprise non-aggregated, entangled networks of individual MWNTs that exhibit non-Newtonian flow behavior. These findings are somewhat unexpected, as EtOH is a poor solvent for PVA. Yet recalling that non-fully hydrated PVA polymers contain PVAc blocks and that EtOH is a good solvent for PVAc, we suggest that re-arrangement of adsorbed chains results in an increased range of the polymer-induced repulsion among polymer-decorated MWNTs and stabilizes the suspended MWNTs. Another aspect of the observations is the apparent dimensional selectivity of solvated PVA polymers towards CB as compared to MWNTs: CB (unlike MWNTs) is readily dispersed in aqueous solutions of PVA. These findings can be rationalized by recalling that polymers adsorbed onto colloidal particles, with a diameter significantly larger than the diameter of the solvated polymer coil (Fig. 9), stretch (due to geometrical limitations) in the direction normal to the surface.14,15 Thus, they exhibit longer-ranged repulsion than those adsorbed onto nano-structures, where polymer dimensions are not modified. The findings offer a simple pathway towards co-processing of PVA and carbonaceous nanostructures in aqueous solutions.
water solutions, as long as the degree of hydration of the polymer is ≤90%. Experimental observations indicate that above a threshold concentration of EtOH (and PVA), long-lived dispersions are formed. The dispersions comprise non-aggregated, entangled networks of individual MWNTs that exhibit non-Newtonian flow behavior. These findings are somewhat unexpected, as EtOH is a poor solvent for PVA. Yet recalling that non-fully hydrated PVA polymers contain PVAc blocks and that EtOH is a good solvent for PVAc, we suggest that re-arrangement of adsorbed chains results in an increased range of the polymer-induced repulsion among polymer-decorated MWNTs and stabilizes the suspended MWNTs. Another aspect of the observations is the apparent dimensional selectivity of solvated PVA polymers towards CB as compared to MWNTs: CB (unlike MWNTs) is readily dispersed in aqueous solutions of PVA. These findings can be rationalized by recalling that polymers adsorbed onto colloidal particles, with a diameter significantly larger than the diameter of the solvated polymer coil (Fig. 9), stretch (due to geometrical limitations) in the direction normal to the surface.14,15 Thus, they exhibit longer-ranged repulsion than those adsorbed onto nano-structures, where polymer dimensions are not modified. The findings offer a simple pathway towards co-processing of PVA and carbonaceous nanostructures in aqueous solutions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Yael-Levi Kalisman from The Center for Nanoscience and Nanotechnology, and The Institute of Life Sciences at the Hebrew University of Jerusalem, Israel, for the cryo-TEM measurements. R. Y.-R. holds the Stanley D. and Nikki Waxberg professorial chair in Advanced Materials. The support of the Israel Science Foundation grant no. 193/18 is acknowledged.References
- B. K. Tan, Y. C. Ching, S. C. Poh, L. C. Abdullah and S. N. Gan, Polymers, 2015, 7, 2205–2222 CrossRef CAS
.
-
C. M. Hassan and N. A. Peppas, Biopolymers·PVA Hydrogels, Anionic Polymerisation Nanocomposites, Springer, 2000, pp. 37–65 Search PubMed
.
- R. N. Olaru, D. M. Vuluga, F. Georgescu, D. Marinescu and M. Dimonie, Sci. Bull. – Univ. “Politeh.” Bucharest, Ser. B, 2010, 72, 77–84 CAS
.
- T. Moritani and Y. Fujiwara, Macromolecules, 1977, 10, 532–535 CrossRef CAS
.
- X. Zhang, T. Liu, T. V. Sreekumar, S. Kumar, V. C. Moore, R. H. Hauge and R. E. Smalley, Nano Lett., 2003, 3, 1285–1288 CrossRef CAS
.
- P. Castell, M. Cano, W. K. Maser and A. M. Benito, Compos. Sci. Technol., 2013, 80, 101–107 CrossRef CAS
.
- J. N. Coleman, U. Khan, W. J. Blau and Y. K. Gun’ko, Carbon, 2006, 44, 1624–1652 CrossRef CAS
.
- R. Shvartzman-Cohen, Y. Levi-Kalisman, E. Nativ-Roth and R. Yerushalmi-Rozen, Langmuir, 2004, 20, 6085–6088 CrossRef CAS
.
- S. Cui, R. Canet, A. Derre, M. Couzi and P. Delhaes, Carbon, 2003, 41, 797–809 CrossRef CAS
.
- L. Léger and C. Creton, Philos. Trans. R. Soc., A, 2008, 366, 1425–1442 CrossRef
.
- E. Raphaël and P. G. De Gennes, J. Phys. Chem., 1992, 96, 4002–4007 CrossRef
.
- B. Vigolo, A. Pénicaud, C. Coulon, C. Sauder, R. Pailler, C. Journet, P. Bernier and P. Poulin, Science, 2000, 290, 1331–1334 CrossRef CAS
.
- C. Mercader, V. Denis Lutard, S. Jestin, M. Maugey, A. Derré, C. Zakri and P. Poulin, J. Appl. Polym. Sci., 2012, 125 Search PubMed
.
- L. Liu, A. H. Barber, S. Nuriel and H. D. Wagner, Adv. Funct. Mater., 2005, 15, 975–980 CrossRef CAS
.
-
J. Brandrup, E. H. Immergut and E. A. Grulke, Polymer handbook, 4th edn, 1999 Search PubMed
.
- I. Szleifer and R. Yerushalmi-Rozen, Polymer, 2005, 46, 7803–7818 CrossRef CAS
.
- R. Shvartzman-Cohen, E. Nativ-Roth, E. Baskaran, Y. Levi-Kalisman, I. Szleifer and R. Yerushalmi-Rozen, J. Am. Chem. Soc., 2004, 126, 14850–14857 CrossRef CAS
.
- F. Du, R. C. Scogna, W. Zhou, S. Brand, J. E. Fischer and K. I. Winey, Macromolecules, 2004, 37, 9048–9055 CrossRef CAS
.
-
A. Fernandez-Nieves and A. M. Puertas, Fluids, Colloids and Soft Materials: An Introduction to Soft Matter Physics, John Wiley & Sons, 2016 Search PubMed
.
- P. Cassagnau, Polymer, 2008, 49, 2183–2196 CrossRef CAS
.
- L. A. Utracki, Polym. Compos., 1986, 7, 274–282 CrossRef CAS
.
-
C. W. Macosko and R. G. Larson, Rheology: principles, measurements, and applications, VCH, New York, NY, 1994 Search PubMed
.
- L. A. Girifalco, M. Hodak and R. S. Lee, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 13104 CrossRef CAS
.
- A. Bar-Hen, C. Bounioux, R. Yerushalmi-Rozen, E. G. Solveyra and I. Szleifer, J. Colloid Interface Sci., 2015, 452, 62–68 CrossRef CAS
.
- M. S. Strano, V. Moore, M. K. Miller, M. J. Allen, E. Haroz, C. Kittrell, R. Hauge and R. E. Smalley, J. Nanosci. Nanotechnol., 2003, 3, 81–86 CrossRef CAS
.
- R. Shvartzman-Cohen, I. Monje, M. Florent, V. Frydman, D. Goldfarb and R. Yerushalmi-Rozen, Macromolecules, 2009, 43, 606–614 CrossRef
.
- S. Kudo, E. Otsuka and A. Suzuki, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 1978–1986 CrossRef CAS
.
- M. Fuchs and K. S. Schweizer, J. Phys.: Condens. Matter, 2002, 14, R239 CrossRef CAS
.
- U. S. Schwarz and S. A. Safran, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2000, 62, 6957 CrossRef CAS
.
-
A. Y. Malkin and A. I. Isayev, Rheology: concepts, methods, and applications, ChemTec, 2006 Search PubMed
.
-
N. Casson, Rheology of disperse systems, Pergamon, Oxford, 1959, pp. 84–104 Search PubMed
.
-
N. Willenbacher and K. Georgieva, Product design and engineering: Formulation of gels and pastes, Wiley-VCH Verlag GmbH & Co. KGaA, 2013, pp. 7–49 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sm01866a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2019 |