Phosphines in artificial photosynthesis: considering different aspects such as chromophores, water reduction catalysts (WRCs), water oxidation catalysts (WOCs), and dyads
Abstract
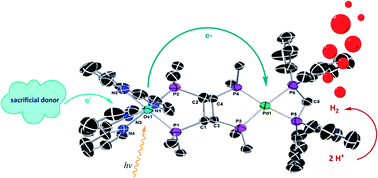
In this review we present the development of mono-, di-, and tetraphosphine ligands for chromophores and water reduction catalysts (WRCs) in artificial photosynthesis (AP) during the last few decades. The use of bis(bidentate) tetraphosphines for dyads is shown. These tetraphosphines can be tailored in order to exploit five-membered ring effects upon chelation, leading to very stable dyads. The aim is to design photocatalytically active systems for sunlight-induced hydrogen production as a sustainable energy harvesting and storage technology of the future, with the help of suitable phosphine ligands. Phosphines play a major role in homogeneous catalysis and the purpose of this review is to show that this is valid for artificial photosynthesis as well. The focus is mainly on originals such as the monophosphine PPh3, the diphosphine xantphos and typical tetraphosphines, e.g. cis,trans,cis-1,2,3,4-tetrakis-(diphenylphosphanyl)cyclobutane (dppcb). Since the phosphorus atom in phosphines is a soft donor within the HSAB concept, especially complexes of soft metals and low oxidation states can be stabilized. Their development and application in a broad range of metal complexes in homogeneous catalysis, as well as their combination in phosphine-based dyads, will be discussed. Due to the creation of a rigid phosphine backbone, several metal complexes could be designed and optimized in respect of their application as chromophores or water reduction catalysts (WRCs). Therefore, in the case of WRCs, the phosphine ligand mainly serves as a stabilizer to improve the catalytic stability, while chromophoric units favour sterically demanding ligands for the advancement of photophysical properties, such as broad absorption spectra, longer-lived excited states and better charge separation, which are desirable for AP. Thus, the photophysical and reduction behaviours during excitation and catalysis were improved steadily. Though the oxophilicity of phosphines is obviously a problem, their possible application in the field of water oxidation catalysts (WOCs) is presented. Metal complexes for AP, incorporating both noble and earth abundant metals, showed long excited state lifetimes and high quantum yields and charge separation when combined with a suitable phosphine ligand. Additionally, higher turnovers and stable systems over more than 240 h could be obtained. This advantage of phosphine ligands over nitrogen containing ligands typically used in artificial photosynthesis is certainly a consequence of enhanced back bonding in phosphine complexes. The storyline of this review is dedicated to this stabilization effect of phosphines outperforming several other classes of ligands.


 Please wait while we load your content...
Please wait while we load your content...