Enhancing the production of hydrogen peroxide from electrocatalytic oxygen reduction reaction by tailoring the electronic states of single-walled carbon nanotubes: a synergistic effect from interior filling and exterior oxidation†
Abstract
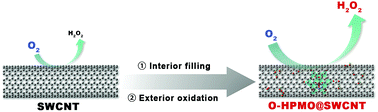
A new strategy is put forward for tailoring the electronic states of single-walled carbon nanotubes (SWCNTs) for the production of hydrogen peroxide from electrocatalytic oxygen reduction reaction (ORR), as achieved by a combination of the interior filling and exterior oxidation. The resultant co-modified SWCNTs obviously outperform those with unitary modifications. This phenomenon is tentatively attributed to the synergistic effect from the interior filling compensating for the electronic conductivity and the exterior oxidation creating additional active sites. These aspects prevent the introduction of excessive defects to the SWCNTs and simultaneously provide additional suitable electronic states to undergo electrocatalytic two-electron ORR.
- This article is part of the themed collection: 2019 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...